
Product Manager:Nick Wilde
1-Fluoropyridinium triflate serves as both an electrophilic fluorinating agent and a one-electron oxidant.

See also: 1-Fluoro-2,4,6-trimethylpyridinium triflate
Recent Literature
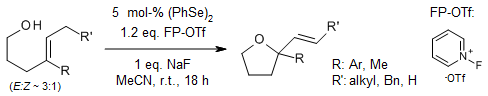
Organoselenium catalysis facilitates the efficient synthesis of oxygen- and nitrogen-containing heterocycles through exo-cyclization under mild conditions using 1-Fluoropyridinium triflate as an oxidant. This method exhibits broad functional group compatibility and high regioselectivity.
R. Guo, J. Huang, H. Huang, X. Zhao, Org. Lett., 2016, 18, 504-507.
https://doi.org/10.1021/acs.orglett.5b03543
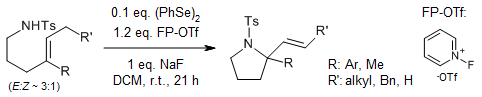
Organoselenium catalysis facilitates the efficient synthesis of oxygen- and nitrogen-containing heterocycles through exo-cyclization under mild conditions using 1-Fluoropyridinium triflate as an oxidant. This method exhibits broad functional group compatibility and high regioselectivity.
R. Guo, J. Huang, H. Huang, X. Zhao, Org. Lett., 2016, 18, 504-507.
https://doi.org/10.1021/acs.orglett.5b03543
Quoted from:
https://www.organic-chemistry.org/chemicals/oxidations/n-fluoropyridinium-triflate.shtm
Aladdin:https://www.aladdinsci.com
