Product Manager:Nick Wilde
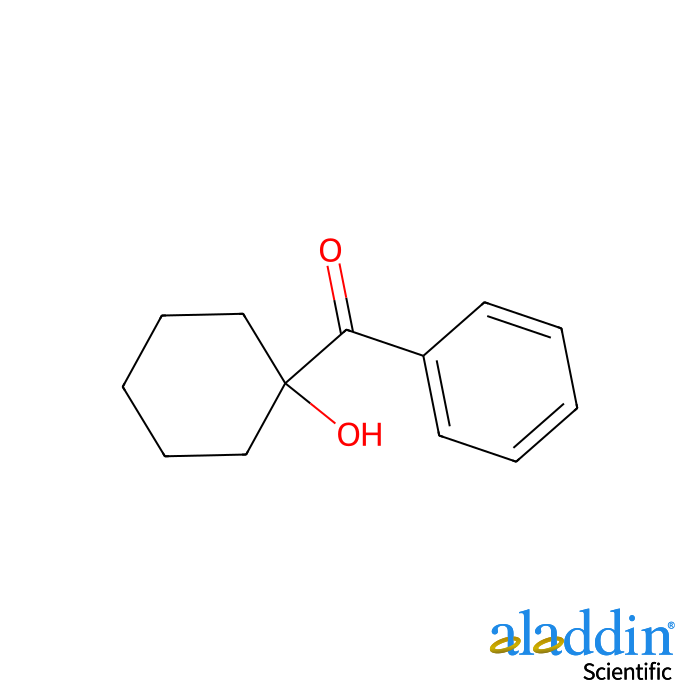
1-Hydroxycyclohexyl phenyl ketone, which is readily accessible on the commercial market, serves as an outstanding hydrogen acceptor in hydrogen - transfer oxidation reactions. For instance, this reagent facilitates the straightforward oxidation of primary alcohols and aldehydes to their corresponding carboxylic acids when sodium hydroxide is present.
Recent Literature
![]()

A metal-free, chemoselective oxidation process transforms primary alcohols and aldehydes into their corresponding carboxylic acids using the inexpensive 1-hydroxycyclohexyl phenyl ketone as the oxidant. This method boasts a user - friendly procedure, delivers high isolated yields, and exhibits remarkable functional group compatibility. It can even operate effectively in the presence of delicate secondary alcohols and tert - butanesulfinamides.
W.-Y. Tan, Y. Lu, J.-F. Zhao, W. Chen, H. Zhang, Org. Lett., 2021, 23, 6648-6653.
https://doi.org/10.1021/acs.orglett.1c02188
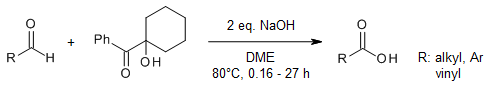
A metal-free, chemoselective oxidation process transforms primary alcohols and aldehydes into their corresponding carboxylic acids using the inexpensive 1-hydroxycyclohexyl phenyl ketone as the oxidant. This method boasts a user - friendly procedure, delivers high isolated yields, and exhibits remarkable functional group compatibility. It can even operate effectively in the presence of delicate secondary alcohols and tert - butanesulfinamides.
W.-Y. Tan, Y. Lu, J.-F. Zhao, W. Chen, H. Zhang, Org. Lett., 2021, 23, 6648-6653.
https://doi.org/10.1021/acs.orglett.1c02188
Quoted from: https://www.organic-chemistry.org/chemicals/oxidations/1-hydroxycyclohexy-phenyl-ketone-1-HCPK.shtm
Aladdin:www.aladdinsci.com

