Product Manager:Nick Wilde
_%E8%8B%B1%E6%96%87.png?access_token=19f85d90-37c1-4477-858b-e9ad8cde59a0)
2-Iodoxybenzoic acid (IBX), the impact-sensitive intermediate in the synthesis of the Dess-Martin periodinane (DMP), is available in a DMSO solution and is used as an oxidizing agent.

Recent Literature

A mild and efficient method for the oxidation of alcohols using 2-iodoxybenzoic acid (IBX) is achieved with β-cyclodextrin catalysis in a water/acetone (86:14) solvent system. The reaction proceeds smoothly at room temperature, affording excellent yields for a variety of alcohols.
K. Surendra, N. Srilakshmi Krishnaveni, M. Arjun Reddy, Y. V. D. Nageswar, K. Rama Rao, J. Org. Chem., 2003, 68, 2058-2059.
https://doi.org/10.1021/jo026751w

K. Surendra, N. Srilakshmi Krishnaveni, M. Arjun Reddy, Y. V. D. Nageswar, K. Rama Rao, J. Org. Chem., 2003, 68, 2058-2059.
https://doi.org/10.1021/jo026751w

Alcohols can be efficiently oxidized to their corresponding carbonyl compounds using 2-iodoxybenzoic acid (IBX) or Dess-Martin periodinane (DMP) in [bmim]BF₄ or [bmim]PF₆ ionic liquids at room temperature. These reactions proceed with excellent yields and high selectivity, exhibiting faster rates in ionic liquids compared to conventional organic solvents. The byproduct, iodosobenzoic acid (IBA), along with the ionic liquid, can be easily recovered and reused.
J. S. Yadav, B. V. S. Reddy, A. K. Basak, A. V. Narsaiah, Tetrahedron, 2004, 60, 2131-2135.
https://doi.org/10.1016/j.tet.2003.12.056
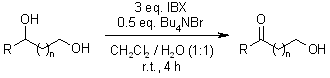
A chemoselective oxidation system consisting of 2-iodoxybenzoic acid (IBX) and n-Bu₄NBr in CH₂Cl₂-water effectively converts secondary alcohols to ketones in good yields. Notably, this method selectively oxidizes secondary hydroxyl groups while preserving primary hydroxyl groups in the same molecule.
C. Kuhakarn, K. Kittigowittana, M. Pohmakotr, V. Reutrakul, Tetrahedron, 2005, 61, 8995-9000.
https://doi.org/10.1016/j.tet.2005.07.051
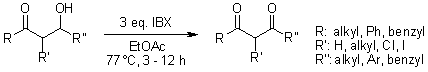
In the oxidation of β-hydroxyketones to β-diketones, 2-iodoxybenzoic acid (IBX) demonstrates remarkable efficiency and operational simplicity, outperforming other conventional oxidants. This method proves effective for both milligram- and gram-scale reactions.
S. L. Bartlett, C. M. Beaudry, J. Org. Chem., 2011, 76, 9852-9855.
https://doi.org/10.1021/jo201810c
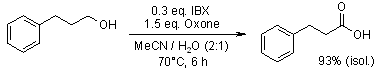
The catalytic oxidation of primary and secondary alcohols has been successfully achieved using 2-iodoxybenzoic acid (IBX) in combination with Oxone as a co-oxidant. This system demonstrates efficient catalytic activity for alcohol oxidations.
A. P. Thottumkara, M. S. Bowsher, T. K. Vinod, Org. Lett., 2005, 7, 2933-2936.
https://doi.org/10.1021/ol050875o

Research demonstrates that 2-iodoxybenzoic acid (IBX) exhibits exceptional efficacy in oxidizing positions adjacent to carbonyl groups and benzylic functionalities, enabling the synthesis of either α,β-unsaturated carbonyl compounds or conjugated aromatic carbonyl structures. Through precise optimization of reaction parameters, this method achieves outstanding selectivity even in complex, multifunctional molecular systems.
K. C. Nicolaou, T. Montagnon, P. S. Baran, Y.-L. Zhong, J. Am. Chem. Soc., 2002, 124, 2245-2258.
https://doi.org/10.1021/ja012127+
K. C. Nicolaou, T. Montagnon, P. S. Baran, Y.-L. Zhong, J. Am. Chem. Soc., 2002, 124, 2245-2258.
https://doi.org/10.1021/ja012127+
K. C. Nicolaou, T. Montagnon, P. S. Baran, Y.-L. Zhong, J. Am. Chem. Soc., 2002, 124, 2245-2258.
https://doi.org/10.1021/ja012127+
The reduction of 2-iodoxybenzoic acid (IBX) to iodosobenzoic acid (IBA) by molecular iodine in dimethyl sulfoxide (DMSO) generates hypoiodous acid (IOH). This reactive species undergoes anti-selective addition to olefins and α,β-unsaturated ketones, producing the corresponding iodohydrins. Remarkably, when conducted in trifluoroacetic acid (TFA)-containing acetonitrile, the same redox system generates iodonium ions that enable efficient electrophilic aromatic iodination.
J. N. Moorthy, K. Senapati, S. Kumar, J. Org. Chem., 2009, 74, 6287-6290.
https://doi.org/10.1021/jo9007892
A mild and highly selective protocol has been developed to directly transform olefins into their corresponding α-bromo ketones by employing the synergistic action of 2-iodoxybenzoic acid (IBX) and tetraethylammonium bromide (1.1 equiv each). This method demonstrates the remarkable synthetic utility of IBX through an operationally simple procedure.
S. S. Deshmukh, K. H. Chaudhari, K. G. Akamanchi, Synlett, 2011, 81-83.
https://doi.org/10.1055/s-0030-1259090
A novel metal-free and environmentally benign catalytic system has been developed for the oxyfluorination of olefins, providing efficient access to synthetically valuable α-fluoroketones. This green methodology demonstrates excellent functional group compatibility, making it particularly attractive for complex molecular synthesis.
Q. Yang, L.-L. Mao, B. Yang, S.-D. Yang, Org. Lett., 2014, 16, 3460-3463.
https://doi.org/10.1021/ol501357w
An efficient and eco-compatible protocol has been developed for 2-iodoxybenzoic acid (IBX)-mediated oxidative rearrangement of 5- and 6-membered cyclic tertiary allylic alcohols to α,β-unsaturated β-disubstituted ketones in DMSO. The method demonstrates excellent compatibility with common protecting groups including acetyl (Ac), methoxymethyl (MOM), and tert-butyldiphenylsilyl (TBDPS) groups.
M. Shibuya, S. Ito, M. Takahashi, Y. Iwabuchi, Org. Lett., 2004, 6, 4303-4306.
https://doi.org/10.1021/ol048210u
Under mild reaction conditions, 2-iodoxybenzoic acid (IBX) efficiently mediates the oxidative cleavage of C-C bonds in 1,3-diols, yielding 1,2-diketones in excellent yields with remarkable selectivity. This transformation proceeds smoothly without requiring harsh conditions or additional catalysts.
J. S. Yadav, S. K. Biswas, R. Srinivas, Synthesis, 2006, 4237-4241.
https://doi.org/10.1055/s-2006-950372
A mild and efficient dehomologation protocol has been developed for α,α-disubstituted acetamides using the hypervalent iodine reagent 2-iodoxybenzoic acid (IBX) in combination with tetraethylammonium bromide (TEAB). This general method enables the one-carbon contraction of acetamides to afford the corresponding ketones in high yields under optimized conditions.
E. V. Bellale, D. S. Bhalarao, K. H. Chaudhari, K. G. Akamanchi, J. Org. Chem., 2008, 73, 9473-9475.
https://doi.org/10.1021/jo801580g
A novel oxidative protocol employing 2-iodoxybenzoic acid (IBX) and tetraethylammonium bromide (TEAB) enables the clean and efficient conversion of primary carboxamides to their corresponding one-carbon shorter nitriles. This method demonstrates remarkable substrate generality and holds significant potential for synthetic applications in organic chemistry.
D. S. Bhalarao, U. S. Mahajan, K. H. Chaudhari, K. G. Akamanchi, J. Org. Chem., 2007, 72, 662-665.
https://doi.org/10.1021/jo0619074
The hypervalent iodine reagent 2-iodoxybenzoic acid (IBX) mediates a remarkably selective oxidative cleavage of inert C(aryl)-N bonds in secondary amides under mild, metal-free conditions, while preserving the C(carbonyl)-N bond intact. This transformation offers a straightforward route to synthesize diverse primary amides with high functional group tolerance.
Z. Zhang, D. Zheng, Y. Wan, G. Zhang, J. Bi, Q. Liu, Q. Liu, T. Liu, L. Shi, J. Org. Chem., 2018, 84, 1369-1376.
https://doi.org/10.1021/acs.joc.7b02880
A practical and mild oxidative desulfurization protocol has been developed using 2-iodoxybenzoic acid (IBX), which efficiently converts readily accessible 1,3-disubstituted thioureas into valuable carbodiimide derivatives. This metal-free transformation proceeds under ambient conditions with excellent atom economy.
P. S. Chaudhari, P. S. Dangate, K. G. Akamanchi, Synlett, 2010, 3065-3067.
https://doi.org/10.1055/s-0030-1259072
A novel and facile transformation of N,N-disubstituted glycylamides into corresponding cyanamides using pentavalent iodine reagents and tetraethylammonium bromide offers advantages such as use of non-toxic reagents, shorter reaction times, and good yields.
K. H. Chaudhari, U. S. Mahajan, D. S. Bhalerao, K. G. Akamanchi, Synlett, 2007, 2815-2818.
https://doi.org/10.1055/s-2007-991093
The reaction of aldehydes, amines, and TMSCN in the presence of 2-iodoxybenzoic acid (IBX) and tetrabutylammonium bromide (TBAB) afforded α-iminonitriles in good to excellent yields under mild conditions. The presence of TBAB is essential for this transformation.
P. Fontaine, A. Chiaroni, G. Masson, J. Zhu, Org. Lett., 2008, 10, 1509-1512.
https://doi.org/10.1055/s-2007-991093
A combination of 2-iodoxybenzoic acid (IBX) and iodine mediates a direct synthesis of β-keto sulfones from alkenes and arenesulfinates in good yields in a one-pot reaction.
N. Samakkanad, P. Katrun, T. Techajaroonjit, S. Hlekhlai, M. Pohmakotr, V. Reutrakul, T. Jaipetch, D. Soorukram, C. Kuhakarn, Synthesis, 2012, 44, 1693-1699.
https://doi.org/10.1055/s-0031-1290952
A combination of 2-iodoxybenzoic acid (IBX) and a catalytic amount of iodine promotes a facile one-pot deacylative sulfonylation reaction of 1,3-dicarbonyl compounds with sodium sulfinates to yield β-carbonyl sulfones in good yields.
P. Katrun, T. Songsichan, D. Soorukram, M. Pohmakotr, V. Reutrakul, C. Kuhakarn, Synthesis, 2017, 49, 1109-1121.
https://doi.org/10.1055/s-0036-1588900
A number of new reactions of 2-iodoxybenzoic acid (IBX) with heteroatom-containing substrates were discovered and their utility was demonstrated. IBX was used for the generation of imines from secondary amines in notably high yields, for the oxidative aromatization of nitrogen heterocycles and for the cleavage of dithianes.
K. C. Nicolaou, C. J. N. Mathison, T. Montagnon, Angew. Chem. Int. Ed., 2003, 42, 4077-4082.
https://doi.org/10.1002/anie.200352076
A series of novel reactions between IBX and heteroatom-containing compounds were uncovered and their synthetic value confirmed. 2-iodoxybenzoic acid (IBX) proved effective for: (i) high-yield imine synthesis from secondary amines, (ii) oxidative aromatization of N-containing heterocycles, and (iii) dithiane ring cleavage.
K. C. Nicolaou, C. J. N. Mathison, T. Montagnon, Angew. Chem. Int. Ed., 2003, 42, 4077-4082.
https://doi.org/10.1002/anie.200352076

2-Iodoxybenzoic acid (IBX) and Dess-Martin periodinane (DMP) effectively convert thiols to thiosulfonates at room temperature. DMP demonstrates superior performance to IBX regarding reaction rate, conversion efficiency, and stoichiometric requirements. Notably, IBX oxidation of benzyl thiols yields thiosulfonates, while DMP produces O-benzyl esters.
A. Chandra, N. Yadav, S. Payra, K. N. Parida, Org. Lett., 2023, 25, 6256-6261.
https://doi.org/10.1021/acs.orglett.3c02017
K. C. Nicolaou, C. J. N. Mathison, T. Montagnon, Angew. Chem. Int. Ed., 2003, 42, 4077-4082.
https://doi.org/10.1002/anie.200352076

2-Iodoxybenzoic acid (IBX) effectively oxidizes diverse epoxides and aziridines as β-cyclodextrin complexes in aqueous media, producing α-hydroxyketones and α-aminoketones in good yields, respectively.
K. Surendra, N. S. Krishnaveni, M. A. Reddy, Y. V. D. Nageswar, K. R. Rao, J. Org. Chem., 2003, 68, 9119-9121.
https://doi.org/10.1021/jo034079c
The 2-iodoxybenzoic acid (IBX)-mediated α-hydroxylation of α-alkynyl carbonyl compounds proceeds efficiently under mildly acidic conditions, selectively affording diverse α-hydroxyketones without formation of dehydrogenation byproducts.
S. F. Kirsch, J. Org. Chem., 2005, 70, 10210-10212.
https://doi.org/10.1021/jo051898j
S. F. Kirsch, J. Org. Chem., 2005, 70, 10210-10212.
https://doi.org/10.1021/jo051898j
A 2-iodoxybenzoic acid (IBX)-mediated selective oxidative cyclization of N-hydroxyalkyl enamines provides a variety of 2,3-disubstituted pyrroles and pyridines in good selectivity. This metal-free method offers use of environmentally friendly reagents, broad substrate scope, mild reaction conditions, and high efficiency.
P. Gao, H.-J. Chen, Z.-J. Bai, M.-N. Zhao, D. Yang, J. Wang, N. Wang, L. Du, Z.-H. Guan, J. Org. Chem., 2020, 85, 7939-7951.
https://doi.org/10.1021/acs.joc.0c00625
A 2-iodoxybenzoic acid (IBX)/tetraethylammonium bromide mediated oxidative cyclization of hydrazide-hydrazones generated in situ from aryl glyoxal and hydrazides enables an efficient and high-yielding protocol for the preparation of α-keto-1,3,4-oxadiazoles under mild conditions in short reaction times.
D. Kumar, M. Pilania, V. Arun, B. Mishra, Synlett, 2014, 25, 1137-1141.
https://doi.org/10.1055/s-0033-1340981
A 2-iodoxybenzoic acid (IBX)-mediated selective oxidative cyclization of N-hydroxyalkyl enamines provides a variety of 2,3-disubstituted pyrroles and pyridines in good selectivity. This metal-free method offers use of environmentally friendly reagents, broad substrate scope, mild reaction conditions, and high efficiency.
P. Gao, H.-J. Chen, Z.-J. Bai, M.-N. Zhao, D. Yang, J. Wang, N. Wang, L. Du, Z.-H. Guan, J. Org. Chem., 2020, 85, 7939-7951.
https://doi.org/10.1021/acs.joc.0c00625
Hantzsch 1,4-dihydropyridines undergo smooth aromatization catalyzed by 2-iodoxybenzoic acid (IBX) to afford the corresponding pyridine derivatives in high yields. All the reactions were carried out in DMSO solvent at 80-85 °C for a period of two to four hours to complete conversion of the substrates.
J. S. Yadav, B. V. S. Reddy, A. K. Basak, G. Baishya, A. V. Narsaiah, Synthesis, 2006, 451-454.
https://doi.org/10.1055/s-2005-918519
The reagent mixture NaI/IBX-SO3K, containing a sulfonylated derivative of 2-iodoxybenzoic acid (IBX), was employed for an oxidative direct conversion of indoles into isatins. A synthetic route toward IBX-SO3K and the X-ray crystal structure of the reagent are presented.
A. Bredenkamp, F. Mohr, S. F. Kirsch, Synthesis, 2015, 47, 1937-1943.
https://doi.org/10.1055/s-0034-1380517
A mild 2-iodoxybenzoic acid (IBX)-catalyzed tandem reaction between readily accessible o-aminobenzylamines and aldehydes provides efficient access to diversely functionalized quinazolines and 3,4-dihydroquinazolines in excellent yields.
S. Hati, S. Sen, Synthesis, 2016, 48, 1389-1398.
https://doi.org/10.1055/s-0035-1560416
A multipathway coupled oxidation/heterocyclization domino strategy enables an efficient synthesis of 2-acylbenzothiazoles from various substrates including arylethenes, arylacetylenes, 2-hydroxy-aromatic ketones and carbinols via four distinct pathways free of metal in one pot.
Y.-p. Zhu, F.-c. Jia, M.-c. Liu, A.-x. Wu, Org. Lett., 2012, 14, 4414-4417.
https://doi.org/10.1021/ol301921t

2-Iodoxybenzoic acid (IBX) in aqueous β-cyclodextrin solution efficiently converts diverse aldehyde and ketone oximes to their parent carbonyl compounds at ambient temperature with exceptional yields.
N. S. Krishnaveni, K. Surendra, Y. V. D. Nageswar, K. R. Rao, Synthesis, 2003, 1968-1969.
https://doi.org/10.1055/s-2003-41448
An efficient and practical protocol has been established for the hydrolysis of thioacetals/thioketals to their corresponding carbonyl compounds using 2-iodoxybenzoic acid (IBX) with β-cyclodextrin (β-CD) in aqueous media under neutral conditions at ambient temperature, affording excellent yields.
N. S. Krishnaveni, K. Surendra, Y. V. D. Nageswar, K. R. Rao, Synthesis, 2003, 2295-2297.
https://doi.org/10.1055/s-2003-41448
Quoted from: https://www.organic-chemistry.org/chemicals/oxidations/ibx-2-iodoxybenzoicacid.shtm
Aladdin:https://www.aladdinsci.com




























