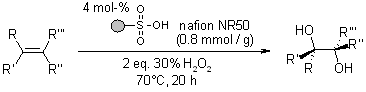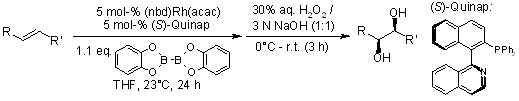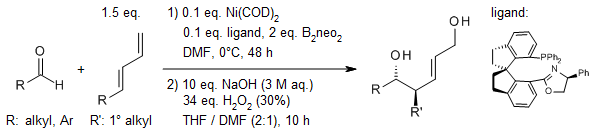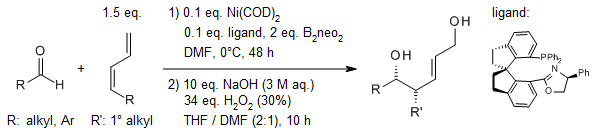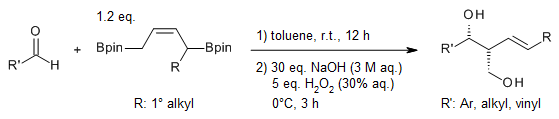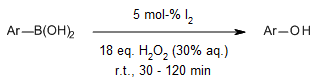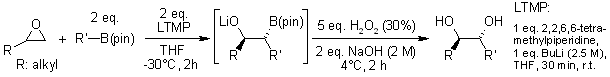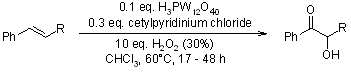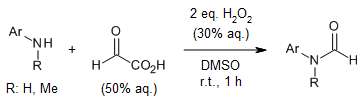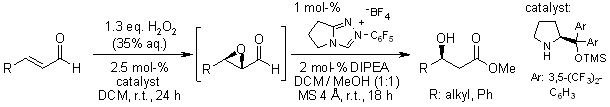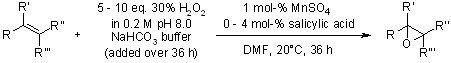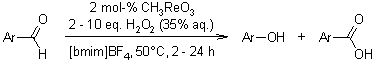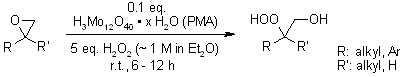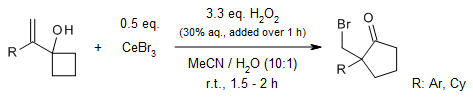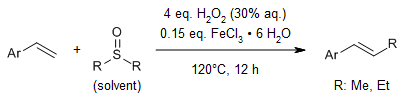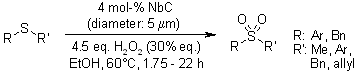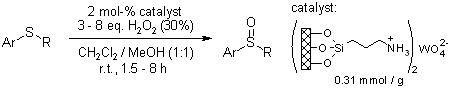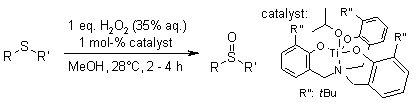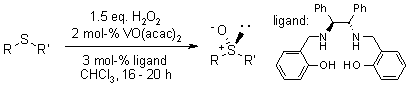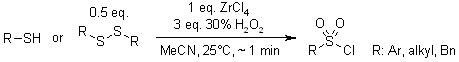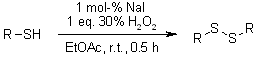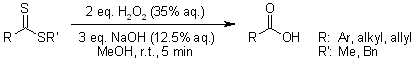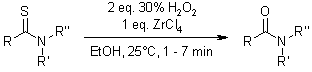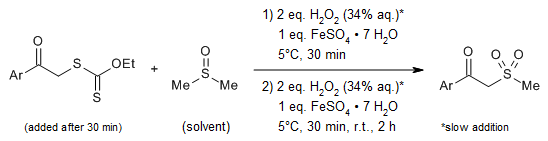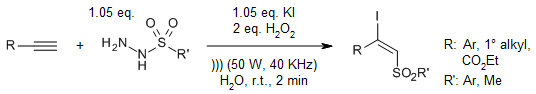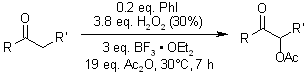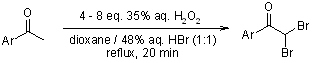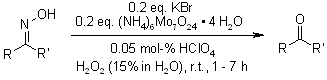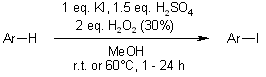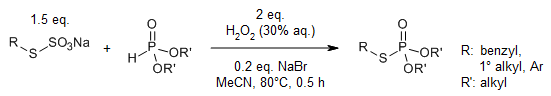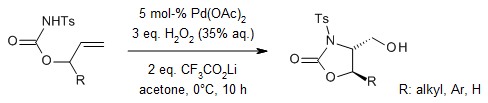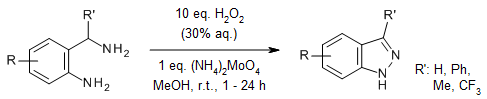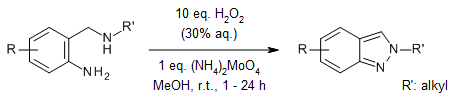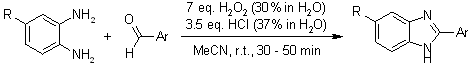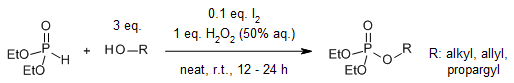Product Manager:Nick Wilde

See also: Hydrogenperoxide urea adduct, Sodium perborate, Sodium percarbonate
Name Reactions
Baeyer-Villiger Oxidation
Follow-up reaction of Brown Hydroboration
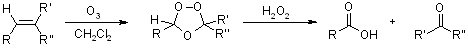
Ozonolysis
Recent Literature
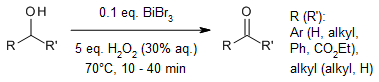
The oxidation of a diverse range of alcohols to carbonyl compounds in commendable yields is efficiently achieved using bismuth tribromide as a catalyst in conjunction with aqueous hydrogen peroxide.
M.-k. Han, S. Kim, S. T. Kim, J. C. Lee, Synlett, 2015, 26, 2434-2436.
https://doi.org/10.1055/s-0035-1560467
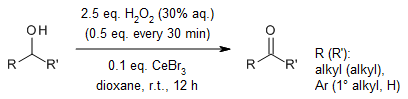
The CeBr3/H2O2 system demonstrates remarkable efficiency in facilitating the green oxidation of secondary and benzylic alcohols into their corresponding carbonyl compounds. This catalytic process operates through a mechanism that entails the formation of a reactive brominating species (RBS). Notably, this RBS exhibits a high degree of oxidation selectivity, favoring the oxidation of secondary alcohols over primary ones.
C. He, F. Ma, W. Zhang, R. Tong, Org. Lett., 2022, 24, 3499-3503.
https://doi.org/10.1021/acs.orglett.2c01133

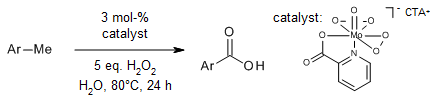
Cost-effective and broadly applicable methodologies have been successfully devised for achieving controlled and predictably selective oxidation of methylarenes and alkylarenes, yielding their corresponding high-value carbonyl compounds. This innovative approach employs a surfactant-stabilized oxodiperoxo molybdenum catalyst in an aqueous medium, utilizing hydrogen peroxide as an environmentally friendly green oxidant. Notably, the process operates efficiently without the need for any external bases, additives, or cocatalysts.
P. Thiruvengetam, D. K. Chand, J. Org. Chem., 2022, 87, 4061-4077.
https://doi.org/10.1021/acs.joc.1c02855
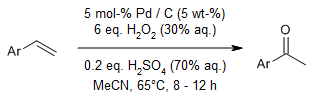
Pd/C demonstrated outstanding catalytic performance in the oxidation of styrene derivatives, efficiently converting them into the corresponding ketones using hydrogen peroxide as the oxidant. This modified Wacker oxidation process is not only cost-effective but also environmentally benign, as it circumvents the necessity for copper salts as co-catalysts.
X. Xia, X. Gao, J. Xu, C. Hu, X. Peng, Synlett, 2017, 28, 607-610.
https://doi.org/10.1055/s-0036-1588656
Under the catalytic influence of a Se/Fe system operating through hybrid reaction mechanisms, the carbon-carbon double bonds in alkenes undergo cleavage, leading to the formation of carbonyl compounds under mild reaction conditions. Given that molecular oxygen (O2) can serve as a partial oxidizing agent in this process, the required amount of H2O2 is significantly reduced, thereby minimizing peroxide residues and enhancing the operational safety of the entire procedure.
X. Li, H. Hua, Y. Liu, L. Yu, Org. Lett., 2023, 25, 6720-6724.
https://doi.org/10.1021/acs.orglett.3c02569
For the selective oxidation of olefins, especially aromatic olefins, into carbonyl compounds, traditional methods often necessitate the use of stoichiometric amounts of toxic oxidants or high-cost catalysts. However, a practical and efficient light-induced oxidation approach has been developed, utilizing H2O2 as a clean and low-toxicity oxidant. This method enables the synthesis of a wide range of carbonyl products in high yields without the need for a catalyst, offering a more sustainable and environmentally friendly alternative.
W. Yu, Z. Zhao, Org. Lett., 2019, 21, 7713-7716.
https://doi.org/10.1021/acs.orglett.9b02569
A sustainable and secure approach has been established for the dihydroxylation of alkenes, operating under organic-solvent-free and metal-free conditions. This method utilizes a resin-supported sulfonic acid catalyst, which offers the added advantage of being effortlessly recyclable.
Y. Usui, K. Sato, M. Tanaka, Angew. Chem. Int. Ed., 2003, 42, 5623-5625.
https://doi.org/10.1002/anie.200352568
Os(VI) can be regenerated to Os(VIII) through a coupled electron-transfer-mediator system that incorporates N-methylmorpholine and a biomimetic flavin. This system facilitates a mild and selective electron-transfer process, enabling the successful cis-dihydroxylation of aliphatic, aromatic, and functionalized olefins. Furthermore, this biomimetic catalytic system demonstrates excellent performance in Sharpless asymmetric dihydroxylation reactions.
S. Y. Jonsson, K. Färnegårdh, J.-E. Bäckvall, J. Am. Chem. Soc., 2001, 123, 1365-1371.
https://doi.org/10.1021/ja0035809
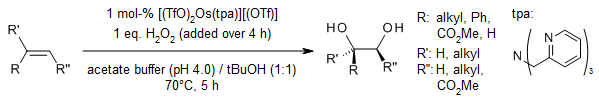
Given the high volatility and significant toxicity of OsO₄, there is a pressing need to develop alternative catalysts for the cis-1,2-dihydroxylation of alkenes. A complex formed by a nitrogen-based tetradentate ligand, tris(2-pyridylmethyl)amine (tpa), and osmium has emerged as a highly efficient recyclable catalyst for the syn-selective dihydroxylation of diverse alkenes in aqueous media, utilizing hydrogen peroxide as the oxidant.
H. Sugimoto, K. Kitayama, S. Mori, S. Itho, J. Am. Chem. Soc., 2012, 134, 19270-19280.
https://doi.org/10.1021/ja309566c
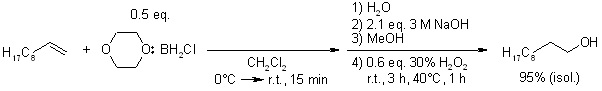
Practical and efficient methods have been devised for synthesizing stable and non-volatile mono- and dichloroborane adducts of dioxane. These adducts are prepared by reacting dioxane-BCl3 with NaBH4 in the presence of catalytic quantities of tri- or tetraglyme. The resulting dioxane-monochloroborane adduct exhibits excellent hydroboration activity, reacting cleanly and rapidly with representative olefins. Subsequent oxidation of the hydroboration products yields the corresponding alcohols in nearly quantitative amounts.
J. V. B. Kanth, H. C. Brown, J. Org. Chem, 2001, 66, 5359-5365.
https://doi.org/10.1021/jo015527o
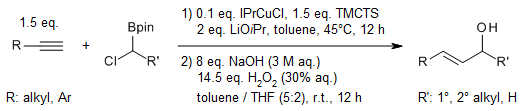
The enantioselective syn addition of bis(catecholato)diboron to simple alkenes, catalyzed by rhodium in the presence of (S)-Quinap as a chiral ligand, leads to the formation of enantioenriched 1,2-diols upon subsequent oxidation. This study delves into the substrate scope of the reaction, explores the underlying reaction mechanism, and examines potential competing pathways that may influence the outcome.
S. Trudeau, J. M. Morgan, M. Shrestha, J. P. Morken, J. Org. Chem., 2005, 70, 9538-9544.
https://doi.org/10.1021/jo051651m
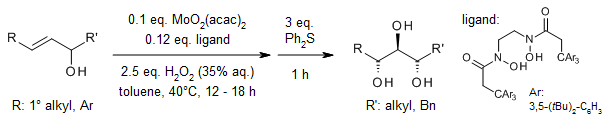
The Mo-catalyzed anti-dihydroxylation of secondary allylic alcohols enables the synthesis of 1,2,3-triols featuring up to three consecutive stereocenters, with remarkable diastereocontrol. This outstanding stereochemical outcome in the final triol products is attributed to the precise control exercised during both the initial epoxidation step, which ensures high diastereoselectivity, and the subsequent in situ hydrolysis, which maintains excellent regioselectivity.
S. Su, C. Wang, Org. Lett., 2019, 21, 2436-2440.
https://doi.org/10.1021/acs.orglett.9b00735
The reductive coupling of terminal alkynes with α-chloro boronic esters offers a highly efficient method for the synthesis of allylic alcohols, exhibiting exceptional regioselectivity (favoring the anti-Markovnikov product) and an impressive E/Z ratio exceeding 200:1. This reaction is versatile, as it can be conducted in the presence of diverse functional groups, and it proceeds with stereospecificity, enabling the reliable and highly selective preparation of chiral allylic alcohols.
A. B. Shaff, L. Yang, M. T. Lee, G. Lalic, J. Am. Chem. Soc., 2023, 145, 24615-24624.
https://doi.org/10.1021/jacs.3c06963
Pt-catalyzed enantioselective addition of bis(pinacolato)diboron (B2(pin)2) to conjugated dienes enables an asymmetric 1,4-dihydroxylation of 1,3-dienes. Dienes which are unable to adopt the S-cis conformation are unreactive. For most substrates, 1,4-addition is the predominant pathway.
H. E. Burks, L. T. Kliman, J. P. Morken, J. Am. Chem. Soc., 2009, 131, 9134-9135.
https://doi.org/10.1021/ja809610h
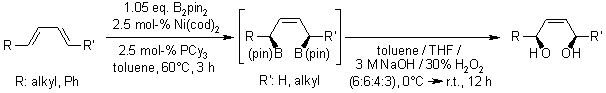
A catalytic stereoselective 1,4-diboration of conjugated dienes with B2(pin)2 and the presence of Ni(cod)2 and PCy3 as the catalyst roceeds efficiently at low catalyst loadings and broadens the substrate scope of current methods for catalytic diene diboration by including internal and sterically hindered. The intermediate allylboronate was oxidized to the stereodefined allylic 1,4-diol.
R. J. Ely, J. P. Morken, Org. Lett., 2010, 12, 4348-4351.
https://doi.org/10.1021/ol101797f
A highly efficient single-step approach for synthesizing valuable homoallylic alcohols has been developed through an enantioselective nickel-catalyzed borylative coupling reaction. This method involves the reaction of 1,3-dienes with aldehydes. The process is facilitated by a chiral spiro phosphine-oxazoline nickel complex, which ensures the products exhibit remarkable diastereoselectivity, E-selectivity, and enantioselectivity.
J.-T. Ma, T. Zhang, B.-Y. Yao, L.-J. Xiao, Q.-L. Zhou, J. Am. Chem. Soc., 2023, 145, 19195-19201.
https://doi.org/10.1021/jacs.3c07697
A highly efficient single-step approach for synthesizing valuable homoallylic alcohols has been developed through an enantioselective nickel-catalyzed borylative coupling reaction. This method involves the reaction of 1,3-dienes with aldehydes. The process is facilitated by a chiral spiro phosphine-oxazoline nickel complex, which ensures the products exhibit remarkable diastereoselectivity, E-selectivity, and enantioselectivity.
J.-T. Ma, T. Zhang, B.-Y. Yao, L.-J. Xiao, Q.-L. Zhou, J. Am. Chem. Soc., 2023, 145, 19195-19201.
https://doi.org/10.1021/jacs.3c07697
The copper-catalyzed 1,4-protoboration of dienylboronates proceeds with high regioselectivity and stereoselectivity, yielding unsymmetrical 1,4-bifunctional allylboron reagents. These reagents then undergo a chemoselective allylboration reaction with aldehydes. Following oxidative workup, the process delivers diol products characterized by excellent diastereoselectivity.
S. Gao, M. Wang, M. Chen, Org. Lett., 2018, 20, 7921-7925.
https://doi.org/10.1021/acs.orglett.8b03483
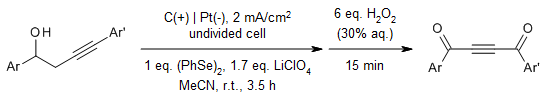
A novel oxidative alkyne translocation strategy enables the synthesis of unsymmetrical but-2-yne-1,4-diones in good yields from readily available homopropargylic alcohols. This transformation occurs through a unique one-pot, sequential process involving electro-oxidative annulation, fragmentation, and chemical selenoxide elimination. Notably, the method exhibits outstanding compatibility with a wide range of functional groups.
A. Halder, D. Maiti, J. Dutta, S. D. Sarkar, Org. Lett., 2023, 25, 7578-7583.
https://doi.org/10.1021/acs.orglett.3c03045

Hydrogen peroxide, recognized as an environmentally friendly oxidant, efficiently facilitates the hydroxylation of arylboronic acids to yield the corresponding products. This reaction proceeds under metal- and base-free aerobic conditions, utilizing a room-temperature ionic liquid (RTIL) as the reaction medium.
E.-J. Shin, G.-T. Kown, S.-H. Kim, Synlett, 2019, 30, 1815-1819.
https://doi.org/10.1055/s-0037-1611894
The employment of aqueous hydrogen peroxide as the oxidizing agent, paired with molecular iodine as the catalyst, presents a gentle and efficient approach for the ipso-hydroxylation of arylboronic acids to produce phenols. This methodology operates under mild conditions, conducted at room temperature with a notably short reaction time, and importantly, proceeds without the need for metals, ligands, or bases.
A. Gogoi, U. Bora, Synlett, 2012, 23, 1079-1081.
https://doi.org/10.1055/s-0031-1290654
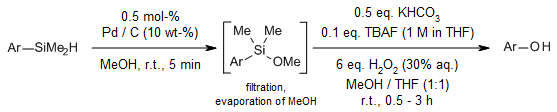
Swift and effective methodologies facilitate the synthesis of phenols through the oxidation of arylhydrosilanes. For electron-rich aromatic compounds, silane activation is achieved by oxidizing them to methoxysilanes, utilizing either homogeneous or heterogeneous transition metal catalysis. Integrating these two oxidation steps into a seamless flow process minimizes the handling and processing of reaction intermediates, streamlining the overall synthesis.
E. J. Rayment, N. Summerhill, E. A. Anderson, J. Org. Chem., 2012, 77, 7052-7060.
https://doi.org/10.1021/jo301363h
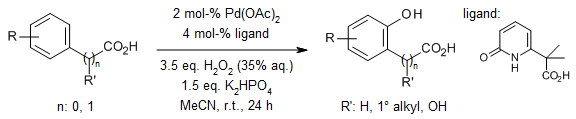
A versatile, bifunctional bidentate carboxyl-pyridone ligand facilitates the palladium-catalyzed C-H hydroxylation of a diverse array of benzoic and phenylacetic acids at room temperature, employing aqueous hydrogen peroxide as the oxidant. The practicality and scalability of this approach are highlighted by a 1 mol scale reaction of ibuprofen, achieved with a remarkably low catalyst loading of just 1 mol % Pd.
Z. Li, H. S. Park, J. X. Qiao, K.-S. Yeung, J.-Q. Yu, J. Am. Chem. Soc., 2022, 144, 18109-18116.
https://doi.org/10.1021/jacs.2c08332
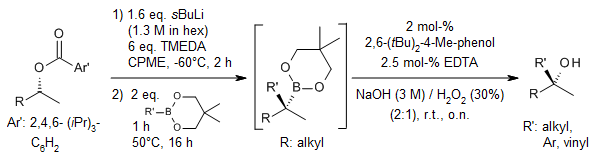
The synergistic use of s-BuLi and TMEDA in CPME at -60 °C facilitates the deprotonation of unactivated, chiral secondary dialkyl TIB esters. The resulting carbanions were subsequently reacted with various neopentyl boronic esters. Following a 1,2-metalate rearrangement and oxidation step, these reactions yielded a diverse array of tertiary alcohols in high yields and with excellent enantiomeric excess (ee). Additionally, the versatility of this methodology was demonstrated through further functional group transformations of the tertiary boronic esters.
A. P. Pulis, D. J. Blair, E. Torres, V. K. Aggarwal, J. Am. Chem. Soc., 2013, 135, 16054-16057.
https://doi.org/10.1021/ja409100y
Lithiated epoxides undergo stereospecific reactions with boronates, resulting in the formation of syn-1,2-diols. This process can be applied iteratively to synthesize triols containing four stereogenic centers.
E. Vedrenne, O. A. Wallner, M. Vitale, F. Schmidt, V. K. Aggarwal, Org. Lett., 2009, 11, 165-168.
https://doi.org/10.1021/ol802651b
Economical and broadly applicable protocols have been devised for the controlled and predictably selective oxidation of methyl-/alkylarenes into their corresponding value-added carbonyl compounds. These methods utilize a surfactant-based oxodiperoxo molybdenum catalyst in an aqueous medium, employing hydrogen peroxide (H2O2) as an environmentally friendly green oxidant. Notably, the reactions proceed without the need for any external bases, additives, or cocatalysts.
P. Thiruvengetam, D. K. Chand, J. Org. Chem., 2022, 87, 4061-4077.
https://doi.org/10.1021/acs.joc.1c02855
A straightforward and mild ketohydroxylation protocol has been developed for the direct conversion of diverse 1-aryl-1-alkenes into acyloins using hydrogen peroxide. This transformation is catalyzed by the cost-effective 12-tungstophosphoric acid/cetylpyridinium chloride system, yielding acyloins in good yields with high regioselectivity.
Y. Zhang, Z. Shen, J. Tang, Y. Zhang, L. Kong, Y. Zhang, Org. Biomol. Chem., 2006, 4, 1478-1482.
https://doi.org/10.1039/b518200j

Diphenyl diselenide facilitates the oxidative degradation of benzoins to benzoic acids under gentle reaction conditions.
H. Cao, T. Chen, C. Yang, J. Ye, X. Zhang, Synlett, 2019, 30, 1683-1687.
https://doi.org/10.1055/s-0037-1611761
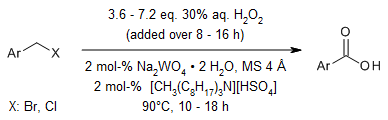
Benzyl chlorides and bromides can undergo direct oxidation to their corresponding benzoic acids through an environmentally safer approach, employing 30% hydrogen peroxide as the oxidant. This reaction is catalyzed by sodium tungstate dihydrate (Na2WO4·2H2O) and facilitated by [CH3(n-C8H17)3N]HSO4 as a phase-transfer catalyst (PTC), all conducted without the use of any organic solvents.
M. Shi, Y.-S. Feng, J. Org. Chem., 2001, 66, 3235-3237.
https://doi.org/10.1021/jo001796n
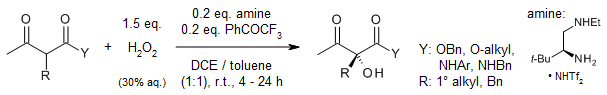
A synergistic dual-catalytic system, combining a primary amine and a ketone, facilitates the asymmetric α-hydroxylation of β-ketocarbonyls using hydrogen peroxide, yielding products in excellent yields and with high enantioselectivity. Notably, this methodology also allows for late-stage hydroxylation of peptidyl amides or chiral esters with remarkable stereoselectivity.
M. Cai, K. Xu, Y. Li, Z. Nie, L. Zhang, S. Luo, J. Am. Chem. Soc., 2021, 143, 1078-1087.
https://doi.org/10.1021/jacs.0c11787
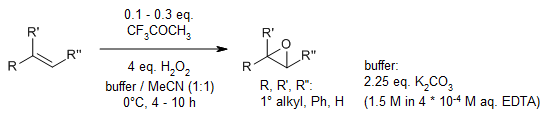
Trifluoroacetone serves as a catalyst for a mild and straightforward epoxidation reaction of various alkenes, achieving good yields using hydrogen peroxide as the primary oxidant under high pH conditions. The utilization of H2O2 as the oxidizing agent notably minimizes the introduction of solvents and salts into the reaction system.
L. Shu, Y. Shi, J. Org. Chem., 2000, 65, 8807-8810.
https://doi.org/10.1021/jo001180y
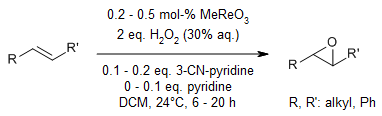
Methyltrioxorhenium (MTO) acts as a catalyst for the epoxidation of alkenes using a 30% aqueous solution of hydrogen peroxide. The incorporation of 1-10 mol% of 3-cyanopyridine enhances the efficiency of the reaction system, leading to high isolated yields of the corresponding epoxides. For alkenes that produce epoxides more susceptible to nucleophilic ring-opening reactions, a combination of 3-cyanopyridine and pyridine is required.
H. Adolfsson, C. Copéret, J. P. Chiang, A. K. Yudin, J. Org. Chem., 2000, 65, 8651-8658.
https://doi.org/10.1021/jo005623+
Introducing SO2F2 gas into a solution containing an olefin, a 30% aqueous hydrogen peroxide solution, and a 2 M aqueous potassium carbonate solution in 1,4-dioxane at room temperature for 1 hour yields the corresponding epoxides in good to excellent yields. This cost-effective, mild, and highly efficient epoxidation system is compatible with a wide range of olefinic substrates, encompassing both electron-rich and electron-deficient variants.
C. Ai, F. Zhu, Y. Wang, Z. Yan, S. Lin, J. Org. Chem., 2019, 84, 11928-11934.
https://doi.org/10.1021/acs.joc.9b01784
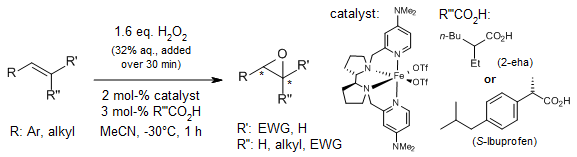
A non-heme iron complex facilitates a highly enantioselective epoxidation of olefins using hydrogen peroxide, aided by catalytic amounts of carboxylic acid additives. The ligand and carboxylic acid work synergistically to promote efficient O-O bond cleavage and generate highly chemo- and enantioselective epoxidizing species. This process yields a diverse array of epoxides in synthetically useful quantities and within short reaction times.
O. Cussó, I. Garcia-Bosch, X. Ribas, J. Lloret-Fillol, M. Costas, J. Am. Chem. Soc., 2013, 135, 14871-14878.
https://doi.org/10.1021/ja4078446
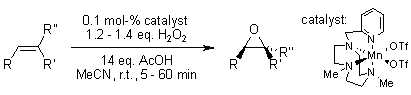
An efficient epoxidation process, applicable to a wide variety of olefins, has been achieved using hydrogen peroxide as the oxidant in the presence of acetic acid and a manganese catalyst that demonstrates unusual chemoselectivity.
I. Garcia-Bosch, X. Ribas, M. Costas, Adv. Synth. Catal., 2008, 351, 348-352.
https://doi.org/10.1002/adsc.200800650
A tungsten-bishydroxamic acid complex promotes a simple, efficient, and environmentally friendly asymmetric epoxidation of allylic, and homoallylic alcohols at room temperature using aqueous hydrogen peroxide as oxidant.
C. Wang, H. Yamamoto, J. Am. Chem. Soc., 2014, 136, 1222-1225.
https://doi.org/10.1021/ja411379e
Homoallylic alcohols were efficiently epoxidized to the corresponding 3,4-epoxy alcohols in excellent yields in the presence of methyltrioxorhenium(MTO) as catalyst, aqueous hydrogen peroxide as the terminal oxidant, and 3-methylpyrazole as an additive. Organic solvent-free conditions accelerate the reaction.
S. Yamazaki, J. Org. Chem., 2012, 77, 9884-9888.
https://doi.org/10.1021/jo301825j
A chiral bisaryl-silyl-protected pyrrolidine functions as a highly selective organocatalyst for epoxidation reactions, utilizing simple oxidizing agents. The broad applicability of this reaction is exemplified by the synthesis of optically active α,β-epoxy aldehydes in high yields and with excellent enantioselectivities. These asymmetric epoxidation reactions can also be conducted under environmentally friendly conditions, such as in water-alcohol mixtures.
M. Marigo, J. Franzen, T. B. Poulsen, W. Zhuang, K. A. Jorgensen, J. Am. Chem. Soc., 2005, 127, 6284-6289.
https://doi.org/10.1021/ja051808s
A suite of 20 chiral epoxides was successfully synthesized with exceptional yields and enantioselectivities within remarkably short reaction times, leveraging hybrid amide-modified Cinchona alkaloid derivatives as catalysts at ultra-low loadings. Notably, the catalyst solution demonstrated remarkable recyclability, retaining its efficacy across 10 consecutive reaction cycles without requiring additional catalyst supplementation.
M. Majdecki, A. Tyszka-Gumkowska, J. Jurczak, Org. Lett., 2020, 22, 8687-8691.
https://doi.org/10.1021/acs.orglett.0c03272
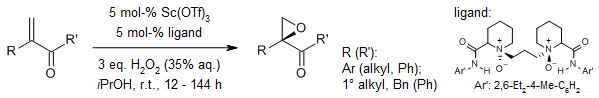
A chiral N,N'-dioxide/Sc³⁺ complex serves as an efficient catalyst for the enantioselective epoxidation of α-substituted vinyl ketones, employing hydrogen peroxide as the oxidant. This process yields key epoxide intermediates critical for the synthesis of diverse triazole-based antifungal agents. The reaction proceeds with high efficiency, delivering products in excellent yields and notable enantioselectivities.
Q. He, D. Zhang, F. Zhang, X. Liu, X. Feng, Org. Lett., 2021, 23, 6795-6800.
https://doi.org/10.1021/acs.orglett.1c02588
An efficient decarboxylative N-formylation protocol for amines, utilizing glyoxylic acid as the formylating agent, yields formamides in high efficiency. This method exhibits broad functional group compatibility under metal-free and base-free conditions, ensuring versatility. Furthermore, its scalability in large-scale experiments and exceptional chemoselectivity underscore its significant potential for practical applications.
J. Wu, Y. Zhang, J. Yang, L. Yu, S. Zhang, J. Zhou, Z. Li, X. Xu, H. Xu, J. Org. Chem., 2023, 88, 13590-13597.
https://doi.org/10.1021/acs.joc.3c01270
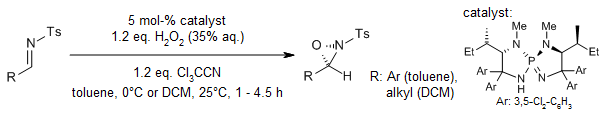
The asymmetric Payne oxidation of N-sulfonyl aldimines, catalyzed by a P-spiro chiral triaminoiminophosphorane, facilitates a highly efficient and enantioselective synthesis of optically active N-sulfonyl oxaziridines. The method’s versatility is underscored by its application in the diastereoselective kinetic oxidation of racemic α-chiral N-sulfonyl imines, showcasing broad substrate compatibility and stereochemical control.
R. Tsutsumi, S. Kim, D. Uraguchi, T. Ooi, Synthesis, 2014, 46, 871-878.
https://doi.org/10.1055/s-0033-1340818
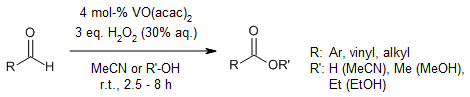
VO(acac)2 efficiently and selectively catalyzes the oxidation of aromatic and aliphatic aldehydes to their corresponding carboxylic acids using hydrogen peroxide as the oxidant. This method is characterized by broad functional group compatibility, straightforward workup procedures, and rapid reaction times. Additionally, the catalytic performance of titania-supported VO(acac)2 in aldehyde oxidation was explored to assess potential improvements in activity or selectivity.
D. Talukdar, K. Sharma, S. K. Bharadwaj, A. J. Thakur, Synlett, 2013, 24, 963-966.
https://doi.org/10.1055/s-0032-1316914
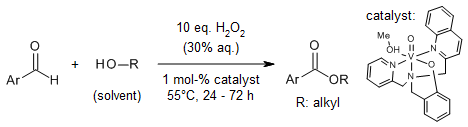
Aromatic aldehydes and benzylic alcohols are efficiently converted into ester derivatives of methanol, ethanol, iso-propanol, n-butanol, sec-butyl alcohol, and propargylic alcohol via a one-pot, additive-free protocol. This transformation utilizes a bench-stable vanadium (V) catalyst and hydrogen peroxide as a green oxidant, delivering products with broad functional group compatibility.
G. Mali, I. Verma, H. Arora, A. Rajput, A. Kumar, R. D. Erande, J. Org. Chem., 2023, 88, 5696-5703.
https://doi.org/10.1021/acs.joc.3c00159
A copper-catalyzed O-methylation of carboxylic acids, employing dimethyl sulfoxide (DMSO) as the methyl source, proceeds with a broad substrate scope and remarkable functional group tolerance. Mechanistic investigations reveal that the reaction involves the generation of a methyl radical from DMSO, providing insights into the reaction pathway.
J. Jia, Q. Jiang, A. Zhao, B. Xu, Q. Liu, W.-P. Luo, C.-C. Guo, Synthesis, 2016, 48, 421-428.
https://doi.org/10.1055/s-0035-1560967
A dual catalytic system combining amino and N-heterocyclic carbene (NHC) catalysts enables a one-pot reaction sequence for the synthesis of β-hydroxy and β-amino esters with high yields and exceptional enantiopurity. This approach utilizes commercially available catalysts at low loadings and facilitates the formation of quaternary stereocenters, demonstrating scalability to gram-level synthesis without the need for inert or anhydrous conditions.
H. Jiang, B. Gschwend, Ł. Albrecht, K. A. Jørgensen, Org. Lett., 2010, 12, 5052-5055.
https://doi.org/10.1021/ol102164y
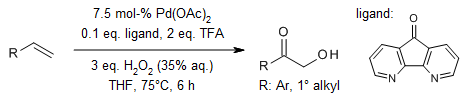
A palladium-catalyzed dioxygenation of simple alkenes provides an environmentally benign route for the rapid synthesis of valuable α-hydroxy ketones, achieving high atom economy.
J. Huang, J. Li, J. Zheng, W. Wu, W. Hu, L. Ouyang, H. Jiang, Org. Lett., 2017, 19, 3354-3357.
https://doi.org/10.1021/acs.orglett.7b01228
An effective epoxidation of lipophilic alkenes using hydrogen peroxide was accomplished with a manganese sulfate/bicarbonate catalytic system in an ionic liquid at room temperature.
K.-H. Tong, K.-Y. Wong, T. H. Chan, Org. Lett., 2003, 5, 3423-3425.
https://doi.org/10.1021/ol035163h
An epoxidation of alkenes using hydrogen peroxide as the terminal oxidant is promoted by catalytic amounts (1.0-0.1 mol %) of manganese(2+) salts, and must be performed using at least catalytic amounts of bicarbonate buffer. Various aryl-substituted, cyclic, and trialkyl-substituted alkenes were epoxidized under these conditions using 10 equiv of hydrogen peroxide, but monoalkyl-alkenes were not. Additives such as sodium acetate and salicylic acid enhanced the rate of the desired epoxidation reaction by 2-3 times. Possible mechanisms for the reaction are discussed.
B. S. Lane, M. Vogt, V. J. DeRosa, K. Burgess, J. Am. Chem. Soc., 2002, 124, 11946-11954.
https://doi.org/10.1021/ja025956j
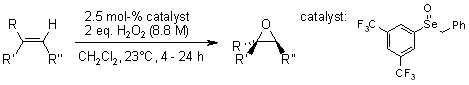
Aryl benzyl selenoxides serve as highly efficient catalysts for the epoxidation of diverse olefinic substrates and the Baeyer-Villiger oxidation of aldehydes and ketones, utilizing hydrogen peroxide as the oxidant.
M. A. Goodman, M. R. Detty, Synlett, 2006, 1100-1104.
https://doi.org/10.1055/s-2006-939692
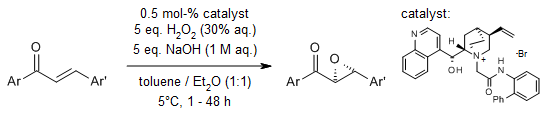
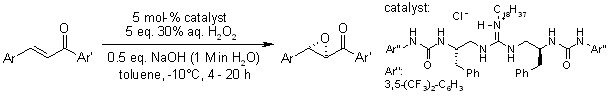
A highly enantioselective catalytic epoxidation of α,β-unsaturated diaryl enones was realized with high chemical yield using aqueous hydrogen peroxide as the oxidant, facilitated by a guanidine-urea bifunctional organocatalyst. The catalyst operates through a cooperative mechanism, wherein the guanidine moiety interacts with H₂O₂ and the urea moiety engages with the enone substrate (or vice versa), enabling precise stereocontrol.
S. Tanaka, K. Nagasawa Synlett, 2009, 667-670.
https://doi.org/10.1055/s-0028-1087811
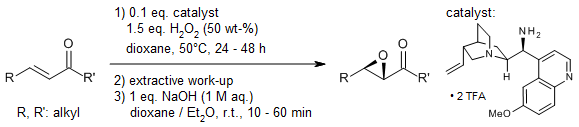
Leveraging cinchona alkaloid-derived primary amines as catalysts and aqueous hydrogen peroxide as the oxidant, highly enantioselective Weitz-Scheffer-type epoxidation and hydroperoxidation of α,β-unsaturated carbonyl compounds are successfully carried out. This catalytic system enables the conversion of acyclic enones, cyclic enones, and α-branched enals with excellent stereocontrol. The reaction intermediates have been rigorously characterized using mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy, while density functional theory (DFT) computations provide mechanistic insights into H₂O₂ activation.
O. Lifchits, M. Mahlau, C. M. Reisinger, A. Lee, C. Farès, I. Polyak, G. Gopakumar, W. Thiel, B. List, J. Am. Chem. Soc., 2013, 135, 6677-6693.
https://doi.org/10.1021/ja402058v
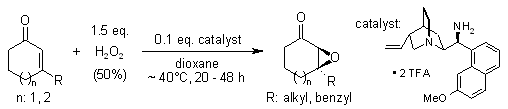
Chiral primary amine salts serve as efficient catalysts for highly enantioselective epoxidations of cyclic enones using hydrogen peroxide as the oxidant.
X. Wang, C. M. Reisinger, B. List, J. Am. Chem. Soc., 2008, 130, 6070-6071.
https://doi.org/10.1021/ja801181u
M. A. Goodman, M. R. Detty, Synlett, 2006, 1100-1104.
https://doi.org/10.1055/s-2006-939692
Aldehydes can be oxidized to form methyl esters when dissolved in methanol and treated with catalytic quantities of vanadium pentoxide along with hydrogen peroxide. This particular method offers several advantages, including mild operating conditions, rapid reaction kinetics, high yields, economic viability, and straightforward product isolation.
R. Gopinath, B. Patel, Org. Lett., 2000, 2, 577-579.
https://doi.org/10.1021/ol990383+
Hydroxylated and methoxylated benzaldehydes and acetophenones can be conveniently and efficiently oxidized to their corresponding phenolic compounds in ionic liquids, specifically [bmim]BF4 and [bmim]PF6, using hydrogen peroxide as the oxidizing agent and methyltrioxorhenium as the catalyst.
R. Bernini, A. Coratti, G. Provenzano, G. Fabrizi, D. Tofani, Tetrahedron, 2005, 61, 1821-1825.
https://doi.org/10.1021/ol990383+
When catalytic quantities of phosphomolybdic acid (PMA) were present, ethereal hydrogen peroxide readily reacted with various epoxides at room temperature. This reaction yielded the corresponding β - hydroxyhydroperoxides in satisfactory amounts.
Y. Li, H.-D. Hao, Y. Wu, Org. Lett., 2009, 11, 2691-2694.
https://doi.org/10.1021/ol900811m
A convenient, safe, and green protocol, that uses oxone/halide and Fenton bromide, achieves a halogenative semipinacol rearrangement at room temperature. The key feature of this method is the green in situ generation of reactive halogenating species from oxidation of halide with oxone or hydrogen peroxide, which produces a nontoxic byproduct .
L. Song, Y. Zhou, H. Liang, H. Li, Y. Lai, H. Yao, R. Lin, R. Tong, J. Org. Chem., 2023, 88, 504-512.
https://doi.org/10.1021/acs.joc.2c02496
Under acidic catalysis, the reaction between β,δ - triketones and hydrogen peroxide proceeds selectively to form tricyclic peroxides in favorable yields. This occurs through the monoperoxidation of the carbonyl groups located at the β - position and the conversion of the δ - carbonyl group into an acetal. Moreover, the peroxides thus produced can be readily separated from the reaction mixture.
A. O. Terent'ev, I. A. Yaremenko, V. V. Chernyshev, V. M. Dembitsky, G. I. Nikishin, J. Org. Chem., 2012, 77, 1833-1842.
https://doi.org/10.1021/jo202437r
The Fe³⁺/hydrogen peroxide system acts as a catalyst to facilitate a regioselective alkylation or arylation process, where alkenes serve as the reaction substrates and sulfoxides act as the alkyl or aryl - donating reagents. This method enables the regioselective synthesis of higher - substituted alkenes, yielding di -, tri -, and even tetra - substituted products. Notably, both aliphatic and aromatic alkenes are compatible with and can effectively take part in this chemical transformation.
M.-D. Su, Y.-F. Liu, Z.-W. Nie, T.-L. Yang, Z.-Z. Cao, H. Li, W.-P. Luo, Q. Liu, C.-C. Guo, J. Org. Chem., 2022, 87, 7022-7032.
https://doi.org/10.1021/acs.joc.2c00047
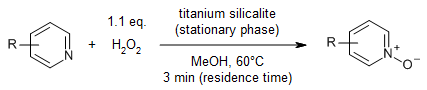
The use of titanium silicalite (TS-1) in a packed-bed microreactor and hydrogen peroxide in methanol as solvent enable the formation of various pyridine N-oxides in very good yields. This flow process is a safer, greener, and more highly efficiency process than using a batch reactor. The device was used for over 800 hours of continuous operation while while the catalyst remained active.
S. Chen, S. Yang, H. Wang, Y. Niu, Z. Zhang, B. Qian, Synthesis, 2022, 54, 3999-4004.
https://doi.org/10.1055/s-0041-1737490
A catalyst-free oxidation of benzylic secondary amines using hydrogen peroxide in MeOH or CH3CN provides a selective access to a variety of C-aryl nitrones in good yields.
A. S. Granato, G. W. Amarante, J. Adrio, J. Org. Chem., 2021, 86, 13817-13823.
https://doi.org/10.1021/acs.joc.1c01888
When sulfides undergo oxidation using 30% hydrogen peroxide, tantalum carbide acts as a catalyst to yield the corresponding sulfoxides in substantial amounts. On the other hand, when niobium carbide is employed as the catalyst in this oxidation process, it efficiently produces the corresponding sulfones. Importantly, both of these catalysts can be readily recovered from the reaction and reused multiple times without any significant loss of their catalytic activity.
M. Kirihara, A. Itou, T. Noguchi, J. Yamamoto, Synlett, 2010, 1557-1561.
https://doi.org/10.1055/s-0029-1219947
When sulfides undergo oxidation using 30% hydrogen peroxide, tantalum carbide acts as a catalyst to yield the corresponding sulfoxides in substantial amounts. On the other hand, when niobium carbide is employed as the catalyst in this oxidation process, it efficiently produces the corresponding sulfones. Importantly, both of these catalysts can be readily recovered from the reaction and reused multiple times without any significant loss of their catalytic activity.
M. Kirihara, A. Itou, T. Noguchi, J. Yamamoto, Synlett, 2010, 1557-1561.
https://doi.org/10.1055/s-0029-1219947
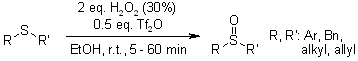
A highly adaptable method oxidizes sulfanes to sulfoxides with precision, avoiding any unwanted overoxidation to sulfones. This is achieved through the combined use of hydrogen peroxide and triflic acid. Moreover, this approach demonstrates remarkable compatibility, tolerating functional groups that are typically sensitive to oxidative conditions.
M. M. Khodaei, K. Bahrami, A. Karimi, Synthesis, 2008, 1682-1684.
https://doi.org/10.1055/s-2008-1067019
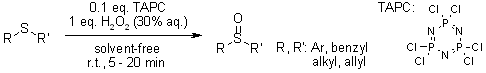
Phosphonitrilic chloride trimer (TAPC) serves as a highly effective catalyst for promoting two key reactions: the oxidation of sulfides and the deoxygenation of sulfoxides. The key benefits of using TAPC in these processes include outstanding product yields, significantly shortened reaction times, straightforward and rapid product isolation, a solvent - free reaction setup, and exceptional chemoselectivity.
K. Bahrami, M. M. Khodaei, M. S. Arabi, J. Org. Chem., 2010, 75, 6208-6213.
https://doi.org/10.1021/jo1011784
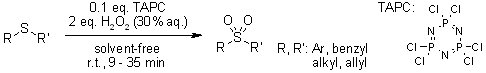
Phosphonitrilic chloride trimer (TAPC) serves as a highly effective catalyst for promoting two key reactions: the oxidation of sulfides and the deoxygenation of sulfoxides. The key benefits of using TAPC in these processes include outstanding product yields, significantly shortened reaction times, straightforward and rapid product isolation, a solvent - free reaction setup, and exceptional chemoselectivity.
K. Bahrami, M. M. Khodaei, M. S. Arabi, J. Org. Chem., 2010, 75, 6208-6213.
https://doi.org/10.1021/jo1011784
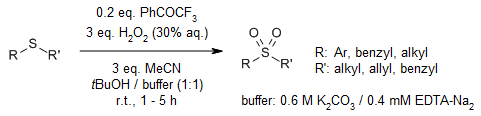
2,2,2-Trifluoroacetophenone as organocatalyst enables a cheap, highly efficient, and selective synthesis of sulfoxides and sulfones starting from sulfides in the presence of hydrogen peroxide as the oxidant. The selectivity depends on the reaction conditions.
E. Voutyritsa, I. Triandafillidi, C. G. Kokotos, Synthesis, 2017, 49, 917-924.
https://doi.org/10.1055/s-0036-1588315
Chiral Brønsted acids confined within specific reaction environments act as catalysts to drive asymmetric oxidation reactions, converting a diverse array of sulfides into sulfoxides using hydrogen peroxide as the oxidant. This newly developed method boasts remarkable versatility, accommodating numerous sulfide substrates, and achieves exceptionally high enantioselectivity. Its performance in terms of both substrate scope and stereocontrol is on par with, and in some cases even surpasses, that of the most advanced metal - based catalytic systems reported to date.
S. Liao, I. Čorić, Q. Wang, B. List, J. Am. Chem. Soc., 2012, 134, 10765-10768.
https://doi.org/10.1021/ja3035637
Drawing inspiration from porphyrin structures, a manganese - catalyzed approach for asymmetric sulfoxidation has been developed. This method allows for the swift oxidation of a wide variety of sulfides, delivering high - yielding products with outstanding enantioselectivities when hydrogen peroxide is used as the oxidizing agent.
W. Dai, J. Li, B. Chen, G. Li, Y. Lv, L. Wang, S. Gao, Org. Lett., 2013, 15, 5658-5661.
https://doi.org/10.1021/ol402612x
At room temperature, a recyclable silica - supported tungstate interphase catalyst facilitates the selective oxidation of an array of aromatic and aliphatic sulfides. When 30% hydrogen peroxide is employed as the oxidizing agent, this process efficiently converts the sulfides into sulfoxides and sulfones, achieving yields that range from good to excellent.
B. Karimi, M. Ghoreishi-Nezhad, J. H. Clark, Org. Lett., 2005, 7, 625-628.
https://doi.org/10.1021/ol047635d
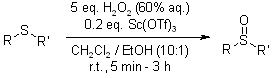
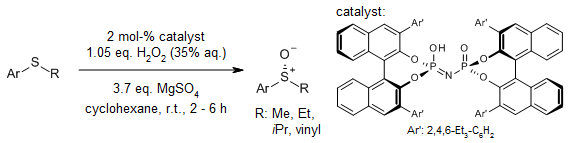
Sc(OTf)3 is an efficient catalyst for the hydrogen peroxide mediated monooxidation of alkyl-aryl sulfides and methyl cysteine containing peptides. The method is high yielding, compatible with many widely used protecting groups, suitable for solid-phase applications and proceeds with minimum over-oxidation.
M. Matteucci, G. Bhalay, M. Bradley, Org. Lett., 2003, 5, 235-237.
https://doi.org/10.1021/ol026947i
A Ti(IV) complex featuring a C3-symmetric triphenolate amine ligand demonstrates air and moisture tolerance, enabling efficient catalytic sulfoxidation reactions at room temperature. Notably, the reaction proceeds without pre-activation when using aqueous hydrogen peroxide as the oxidant.
M. Mba, L. J. Prins, G. Licini, Org. Lett., 2007, 9, 21-24.
https://doi.org/10.1021/ol062395i
The reported system achieves exceptionally high enantiomeric excess (ee) and yield simultaneously, resulting from an efficient initial asymmetric oxidation followed by a highly selective kinetic resolution. This makes it a highly practical method for the asymmetric oxidation of simple alkyl aryl sulfides.
C. Drago, L. Caggiano, R. F. W. Jackson, Angew. Chem. Int. Ed., 2005, 44, 7221-7223.
https://doi.org/10.1002/anie.200503046
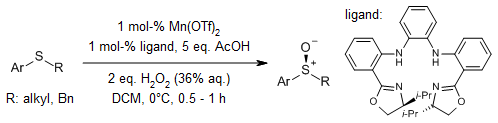
A chiral Fe(salan) complex efficiently catalyzes the asymmetric oxidation of sulfides-including both alkyl aryl sulfides and diverse methyl alkyl sulfides—using aqueous hydrogen peroxide as the oxidant, even in the absence of surfactant. The reaction proceeds with excellent enantioselectivity, yielding the corresponding sulfoxides in high optical purity.
H. Egami, T. Katsuki, J. Am. Chem. Soc., 2007, 129, 8940-8941.
https://doi.org/10.1021/ja071916+
A chiral vanadium-salan complex demonstrates remarkable catalytic efficiency for the asymmetric oxidation of sulfides to chiral sulfoxides using hydrogen peroxide, achieving both excellent yields and high enantioselectivity. Additionally, this vanadium-salan system effectively catalyzes the kinetic resolution of racemic sulfoxides.
J. Sun, C. Zhu, Z. Dai, M. Xang, Y. Pan, H. Hu, J. Org. Chem., 2004, 69, 8500-8503.
https://doi.org/10.1021/jo040221d
LiNbMoO6 demonstrates excellent chemoselectivity in catalyzing the sulfur oxidation of electron-rich allylic sulfides, while completely avoiding epoxidation of their double bonds. By precisely controlling the stoichiometric amount of hydrogen peroxide oxidant, selective oxidation to either sulfoxides or sulfones can be achieved. The reaction shows remarkable functional group tolerance, accommodating hydroxyl, formyl, THP ether, and TBDMS ether groups.
S. Choi, J.-D. Yang, M. Ji, H. Choi, M. Kee, K.-H. Whn, S.-H. Byeon, W. Baik, S. Koo, J. Org. Chem., 2001, 66, 8154-8159.
https://doi.org/10.1021/jo016013s
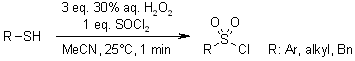
The H2O2/SOCl2 reagent system enables highly efficient oxidative chlorination of thiol derivatives, directly converting them to sulfonyl chlorides with exceptional reactivity. These sulfonyl chlorides can subsequently react with amines to afford the corresponding sulfonamides in excellent yields within remarkably short reaction times.
K. Bahrami, M. M. Khodaei, M. Soheilizad, J. Org. Chem., 2009, 74, 9287-9291.
https://doi.org/10.1021/jo901924m
The ZrCl4/H2O2 system serves as a highly effective oxidative chlorination reagent, enabling the direct conversion of both thiols and disulfides to corresponding sulfonyl chlorides with excellent purity. This method offers significant advantages including high product yields, remarkably fast reaction kinetics, mild operating conditions, and the elimination of hazardous reagents.
K. Bahrami, M. M. Khodaei, M. Soheilizad, Synlett, 2009, 2773-2776.
https://doi.org/10.1055/s-0029-1217989
Thiols undergo efficient oxidative coupling to disulfides when treated with hydrogen peroxide in the presence of catalytic iodide (I⁻) or molecular iodine (I2) as the catalyst.
M. Kirihara, Y. Asai, S. Ogawa, T. Nouchi, A. Hatano, Y. Hirai, Synthesis, 2007, 3286-3289.
https://doi.org/10.1055/s-2007-990800
We present simple, mild, and eco-friendly protocols for the direct transformation of dithioesters into carboxylic acids or esters using hydrogen peroxide under alkaline conditions.
F. Grellepois, C. Portella, Synthesis, 2008, 3443-3446.
https://doi.org/10.1055/s-0028-1083190
The H2O2-ZrCl4 reagent system provides an efficient and versatile approach for converting thioamides to amides, achieving excellent chemoselectivity within short reaction times. This method features a straightforward workup procedure that eliminates the need for toxic solvents.
K. Bahrami, M. M. Khodaei, Y. Tirandaz, Synthesis, 2009, 369-371.
https://doi.org/10.1055/s-0028-1083314
Xanthates react with DMSO in the presence of Fenton's reagent under exceptionally mild conditions to afford diverse β-keto sulfones in excellent yields.
P. N. Chalikidi, M. G. Uchuskin, I. V. Trushkov, V. T. Abaev, O. V. Serdyuk, Synlett, 2018, 29, 571-575.
https://doi.org/10.1055/s-0036-1589151
An efficient ultrasound-assisted synthesis of (E)-β-iodovinyl sulfones has been developed, employing alkynes, sulfonyl hydrazides, KI, and hydrogen peroxide to afford products in high yields. This protocol is characterized by its remarkable reaction speed and synthetic efficiency.
C. Zhou, X. Zeng, Synthesis, 2021, 53, 4614-4620.
https://doi.org/10.1055/a-1559-3346
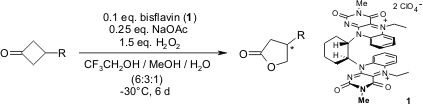
We report the synthesis of a planar-chiral bisflavin catalyst (1) and demonstrate its successful application in asymmetric Baeyer-Villiger oxidations.
S. Murahashi, S. Ono, Y. Imada, Angew. Chem. Int. Ed., 2002, 41, 2366-2368.
https://doi.org/10.1002/1521-3773(20020703)41:13%3C2366::AID-ANIE2366%3E3.0.CO;2-S
An efficient and cost-effective α-acetoxylation of ketones has been developed, employing iodobenzene as the catalyst with acetic anhydride and 30% aqueous hydrogen peroxide as the co-oxidants, providing α-acetoxy ketones in high yields.
J. Sheng, Y. Li, M. Tang, B. Gao, G. Huang, Synthesis, 2007, 1165-1168.
https://doi.org/10.1055/s-2007-965984
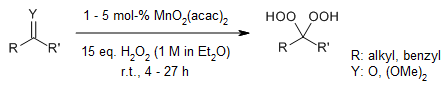
The MoO2(acac)2-catalyzed reaction of ketones, ketals, and epoxides with ethereal hydrogen peroxide enables highly efficient and chemoselective synthesis of corresponding hydroperoxides in excellent yields.
X. An, Q. Zha, Y. Wu, Org. Lett., 2019, 21, 1542-1546.
https://doi.org/10.1021/acs.orglett.9b00425
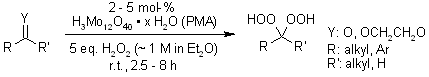
Phosphomolybdic acid (PMA) efficiently catalyzes the conversion of ketones and ketals to their corresponding gem-dihydroperoxides using ethereal H2O2 at room temperature, delivering excellent yields.
Y. Li, H.-D. Hao, Q. Zhang, Y. Wu, Org. Lett., 2009, 11, 1615-1618.
https://doi.org/10.1021/ol900262t
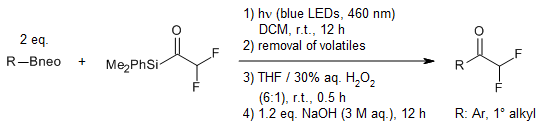
A visible-light-mediated transformation of commercially accessible organoboronic esters and fluoroalkyl acylsilanes provides a versatile platform for the divergent synthesis of fluoroalkyl ketones. The reaction pathway can be selectively controlled: under basic conditions, the organoboronate intermediates undergo deboronative fluoride elimination, whereas in the presence of peroxide, the process favors a fluoroalkyl 1,2-migration.
G. Zhou, Z. Guo, S. Liu, X. Shen, J. Am. Chem. Soc., 2024, 146, 4026-4035.
https://doi.org/10.1021/jacs.3c12150
The H2O2-HBr aqueous system enables rapid dibromination of 1-arylethanones and related compounds in dioxane, selectively replacing two hydrogen atoms in the methyl group with bromine. When the aromatic ring bears electron-donating substituents, concomitant aromatic bromination is observed.
A. O. Terent'ev, S. V. Khodykin, I. B. Krylov, Y. N. Ogibin, G. I. Nikishin, Synthesis, 2006, 1087-1092.
https://doi.org/10.1055/s-2006-926386
A mild and efficient bromination protocol for active methylene compounds has been developed, employing KBr/HCl/H2O2 as the brominating system at ambient temperature to achieve high-yielding and chemoselective transformations.
M. Kirihara, S. Ogawa, T. Noguchi, K. Okubo, Y. Monma, I. Shimizu, R. Shimosaki, A. Hatano, Y. Hirai, Synlett, 2006, 2287-2289.
https://doi.org/10.1055/s-2006-948207
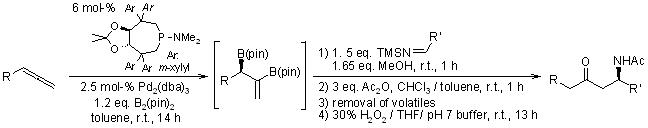
A palladium-catalyzed enantioselective diboration/protonation cascade of prochiral allenes with primary imines generates versatile vinyl boronate intermediates. These intermediates can be oxidatively transformed into enantioenriched Mannich products or, alternatively, converted to chiral homoallylic amines via protonation followed by Suzuki cross-coupling.
J. D. Sieber, J. P. Morken, J. Am. Chem. Soc., 2006, 128, 74-75.
https://doi.org/10.1021/ja057020r
A facile and environmentally benign protocol has been developed for the cleavage of various ketoximes and aldoximes to their parent carbonyl compounds in aqueous media. This transformation employs catalytic KBr/(NH₄)₆Mo₇O₂₄·4H₂O with H2O2 as the oxidant, proceeding under mild conditions with high efficiency.
N. C. Ganguly, S. K. Barik, Synthesis, 2008, 425-428.
https://doi.org/10.1055/s-2008-1032035
A highly selective and efficient oxidative iodination system for electron-rich arenes has been developed, employing stoichiometric KI (1 equiv) and H2O2 (2 equiv, 30%) in methanol under strong acidic conditions.
J. Iskra, S. Stavber, M. Zupan, Synthesis, 2004, 1869-1873.
https://doi.org/10.1055/s-2004-829136
An efficient and environmentally benign NaBr-catalyzed coupling of Bunte salts with phosphonates has been developed, utilizing H2O2 (30%) as the oxidant under acidic conditions to afford thiophosphates in high yields.
C. Min, R. Zhang, Q. Liu, S. Lin, Z. Yan, Synthesis, 2018, 50, 2027-2030.
https://doi.org/10.1055/s-0037-1609556
A ligand-controlled γ-C(sp³)-H selective hydroxylation protocol has been developed for primary amines, piperidines, and morpholines using aqueous hydrogen peroxide as a sustainable oxidant, delivering monohydroxylated products with exceptional selectivity. This versatile method enables direct access to diverse γ-amino alcohols, β-amino acids, and azetidines.
Z. Li, J.-Q. Yu, J. Am. Chem. Soc., 2023, 145, 25948-25953.
https://doi.org/10.1021/jacs.3c09340
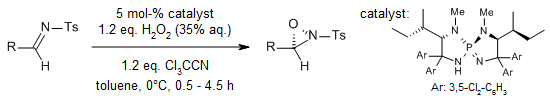
A P-spiro chiral triaminoiminophosphorane-catalyzed, highly enantioselective Payne-type oxidation of N-sulfonyl imines has been developed. This efficient protocol employs an hydrogen peroxide/trichloroacetonitrile co-oxidation system, demonstrating exceptional substrate scope and distinctive chemoselectivity.
D. Uraguchi, R. Tsutsumi, T. Ooi, J. Am. Chem. Soc., 2013, 135, 8161-8164.
https://doi.org/10.1021/ja403491j
A green and practical protocol facilitates the high-yielding synthesis of 2-imidazolines through the reaction of aldehydes with ethylenediamine. This efficient method employs H2O2 as the oxidant along with NaI and anhydrous MgSO4, operating under mild and environmentally benign conditions.
G.-y. Bai, K. Xu, G.-f. Chen, Y.-h. Yang, T.-y. Li, Synthesis, 2011, 1599-1603.
https://doi.org/10.1055/s-0030-1259992
A 2,2,2-trifluoroacetophenone-catalyzed oxidative cyclization of allyloximes with H2O2 as the terminal oxidant provides an environmentally benign and efficient route to isoxazolines. This method demonstrates broad substrate scope, accommodating diverse aromatic and aliphatic substitutions while delivering products in high yields.
I. Triandafillidi, C. G. Kokotos, Org. Lett., 2017, 19, 106-109.
https://doi.org/10.1021/acs.orglett.6b03380
A palladium-catalyzed intramolecular aminohydroxylation via H2O2-mediated oxidative cleavage of alkyl C-Pd bonds has been developed, efficiently producing diverse heterocycles with high yields and exceptional diastereoselectivity. The resulting products serve as versatile intermediates for the synthesis of 2-amino-1,3-diols and 3-hydroxyamino acids. Mechanistic investigations suggest the involvement of an Sₙ2-type water attack at a high-valent palladium center.
H. Zhu, P. Chen, G. Liu, J. Am. Chem. Soc., 2014, 136, 1766-1769.
https://doi.org/10.1021/ja412023b
Candida antarctica lipase B (CAL-B) efficiently catalyzes the H2O2-mediated oxidative ring expansion of furfuryl alcohols under mild conditions, affording functionalized pyranones. This enzymatic strategy is equally applicable to N-protected furfurylamines, enabling the synthesis of valuable piperidinone derivatives through analogous rearrangement.
F. Blume, P. Sprengart, J. Deska, Synlett, 2018, 29, 1293-1296.
https://doi.org/10.1055/s-0036-1591889
An oxidative N-N bond-forming cyclization of readily accessible 2-aminomethyl-phenylamines has been developed, providing direct access to all three tautomeric forms of indazoles. This method selectively delivers pharmaceutically relevant 2-substituted 2H-indazoles—privileged scaffolds in drug discovery—along with the less explored 3H-indazole variants.
A. S. Toledano, J. Bitai, D. Covini, J. Karolyi-Oezguer, C. Dank, H. Berger, A. Gollner, Org. Lett., 2024, 26, 1229-1232.
https://doi.org/10.1021/acs.orglett.4c00036
An oxidative N-N bond-forming cyclization of readily accessible 2-aminomethyl-phenylamines has been developed, providing direct access to all three tautomeric forms of indazoles. This method selectively delivers pharmaceutically relevant 2-substituted 2H-indazoles—privileged scaffolds in drug discovery—along with the less explored 3H-indazole variants.
A. S. Toledano, J. Bitai, D. Covini, J. Karolyi-Oezguer, C. Dank, H. Berger, A. Gollner, Org. Lett., 2024, 26, 1229-1232.
https://doi.org/10.1021/acs.orglett.4c00036
A practical method for synthesizing 2-substituted benzimidazoles and benzothiazoles features short reaction times, scalability for large-scale production, straightforward and rapid product isolation, exceptional chemoselectivity, and high yields as key benefits.
K. Bahrami, M. M. Khodaei, F. Naali, J. Org. Chem., 2008, 73, 6835-6837.
https://doi.org/10.1021/jo8010232
A straightforward and efficient one-pot method for synthesizing substituted benzimidazoles involves the condensation of o-phenylenediamines with aryl aldehydes using H2O2 and HCl in acetonitrile at room temperature. This approach offers rapid reaction times, simple and fast product isolation, and high yields.
K. Bahrami, M. M. Khodaei, I. Kavianinia, Synthesis, 2007, 417-427.
https://doi.org/10.1055/s-2007-965878
An eco-friendly and highly efficient protocol enables the metal-free conversion of α-hydroxy N-arylamides to isatins using hydrogen peroxide as the oxidant, achieving excellent yields. This practical method features a broad substrate scope and mild reaction conditions.
J. Li, X. Cheng, X. Ma, G. Lv, Z. Zhan, M. Guan, Y. Wu, Synlett, 2016, 27, 2485-2488.
https://doi.org/10.1055/s-0035-1562517
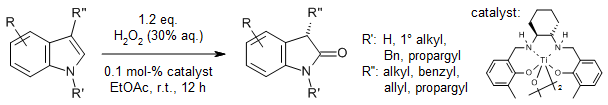
The preparation of chiral 3-monosubstituted oxindoles presents a notable synthetic challenge owing to their propensity for racemization. A titanium-catalyzed chemo- and enantioselective indole oxidation under mild conditions, employing environmentally benign H2O2 as the terminal oxidant, affords a wide array of these valuable compounds in high yields with excellent enantiomeric excess (ee). This transformation demonstrates remarkable functional group compatibility.
S. Li, X. Liu, C.-H. Tung, L. Liu, J. Am. Chem. Soc., 2023, 145, 27120-27130.
https://doi.org/10.1021/jacs.3c11742
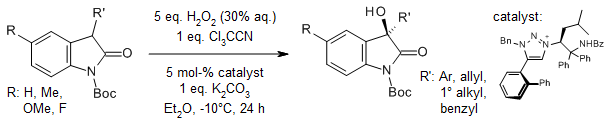
Peroxy trichloroacetimidic acid, generated in situ from hydrogen peroxide solution and trichloroacetonitrile, serves as an effective electrophilic oxygenating reagent. This system enables direct enantioselective α-hydroxylation of oxindoles when using a chiral 1,2,3-triazolium salt as a phase-transfer catalyst.
K. Ohmatsu, Y. Ando, T. Ooi, Synlett, 2017, 28, 1291-1294.
https://doi.org/10.1055/s-0036-1558958
A straightforward method enables the deprotection of 1,3-dithianes and 1,3-dithiolanes using 30% aqueous hydrogen peroxide activated by iodine catalyst (5 mol%) in water with sodium dodecyl sulfate (SDS) under near-neutral conditions. This protocol demonstrates excellent compatibility with various phenol and amino protecting groups while completely avoiding overoxidation.
N. G. Ganguly, S. K. Barik, Synthesis, 2009, 1393-1399.
https://doi.org/10.1055/s-0028-1088023
A mild and efficient phosphorylation method enables the synthesis of diverse phosphoramidates, phosphite triesters, and sulfoximine-derived phosphoramidates from amines, alcohols, and sulfoximines. This catalytic system employs molecular iodine as the catalyst with hydrogen peroxide serving as the sole oxidant under optimized reaction conditions.
J. Dhineshkumar, K. R. Prabhu, Org. Lett., 2013, 15, 6062-6065.
https://doi.org/10.1021/ol402956b
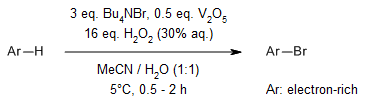
Vanadium pentoxide efficiently catalyzes the bromination of organic substrates using tetrabutylammonium bromide with hydrogen peroxide, enabling selective bromination of aromatic compounds. This method features mild reaction conditions, excellent selectivity, high yields, rapid reaction rates.
U. Bora, G. Bose, M. K. Chaudhuri, S. S. Dhar, R. Gopinath, A. T. Khan, B. K. Patel, Org. Lett., 2000, 2, 247-249.
https://doi.org/10.1021/ol9902935
Quoted from:https://www.organic-chemistry.org/chemicals/oxidations/hydrogenperoxide.shtm
Aladdin:https://www.aladdinsci.com