%EF%BC%88Fe(Cl)3%EF%BC%89_%E8%8B%B11.png?access_token=cd01cffe-565d-478e-b65d-11e16a7a3c2a)
Iron(III) chloride is a mild oxidizing agent.

Recent Literature

Palladium (Pd), ruthenium (Ru), and iron (Fe) catalysts facilitate a versatile method for synthesizing 2-substituted pyrroles, delivering overall good yields while generating only water and ethylene as by-products. The process begins with two consecutive Pd-catalyzed monoallylations of amines using allylic alcohols. Subsequently, Ru-catalyzed ring-closing metathesis of the diallylated amines yields pyrrolines in high efficiency. Finally, treatment with iron(III) chloride enables selective aromatization of the intermediate pyrrolines.
A.Bunrit, S. Sawadjoon, S. Tšupova, P. J. R. Sjöberg, J. S. M. Samec, J. Org. Chem., 2016, 81, 1450-1460.
https://doi.org/10.1021/acs.joc.5b02581
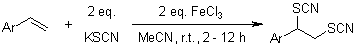
Iron(III) chloride acts as an oxidizing agent to convert potassium thiocyanate into the corresponding radical species, which subsequently undergoes addition to nucleophilic olefins. This reaction efficiently yields dithiocyanate derivatives under mild conditions, offering excellent yields and chemoselectivity. The application of ferric chloride renders this synthetic approach straightforward, user-friendly, and highly practical.
J. S. Yadav, B. V. S. Reddy, M. K. Gupta, Synthesis, 2004, 1983-1986.
https://doi.org/10.1055/s-2004-829150
Quoted from: https://www.organic-chemistry.org/chemicals/oxidations/ferricchloride.shtm
Aladdin:https://www.aladdinsci.com
