%E4%B9%9D%E6%B0%B4%E5%90%88%E7%89%A9_%E4%B8%AD.png?access_token=b44bb54c-877e-4d1f-9424-cba2560ab262)
Product Manager:Nick Wilde

Iron nitrate nonahydrate is a strong oxidant that decomposes with evolution of nitrous fumes.
Recent Literature
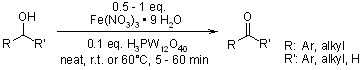
Keggin-type heteropoly acids demonstrated exceptional catalytic efficiency in facilitating the rapid and specific oxidation of diverse hydroxy groups into their respective carbonyl derivatives. This transformation was achieved using ferric nitrate as the oxidizing agent, operating under gentle, solvent-free reaction conditions.
H. Firouzabadi, N. Iranpoor, K. Amani, Synthesis, 2003, 408-412.
https://doi.org/10.1055/s-2003-37343
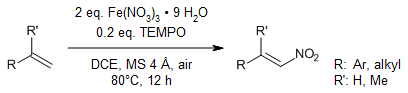
The combination of ferric nitrate and catalytic TEMPO serves as an effective reagent system for the regio- and stereoselective nitration of a wide range of aromatic, aliphatic, and heteroaromatic olefins. This mild and straightforward reaction protocol enables the production of nitroolefins in yields suitable for preparative applications, with remarkable E-selectivity.
T. Naveen, S. Maity, U. Sharma, D. Maiti, J. Org. Chem., 2013, 78, 5949-5954.
https://doi.org/10.1021/jo400598p
Quoted
from:https://www.organic-chemistry.org/chemicals/oxidations/ferricnitrate.shtm
