
Product Manager:Nick Wilde
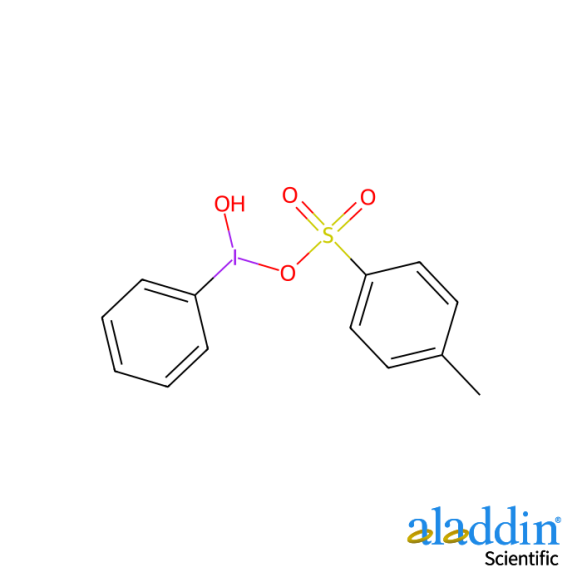
Hydroxy(tosyloxy)iodobenzene (HTIB) is a commercially available reagent widely used for the phenyliodination and oxytosylation of various organic substrates. Typical transformations include: the conversion of ketones to α-tosyloxy ketones and the syn-addition to alkenes yielding 1,2-ditosyloxyalkanes.
A recently developed synthetic approach provides convenient access to Koser's Reagent and its derivatives.

E. A. Merritt, V. M. T. Carneiro, L. F. Silva Jr., B. Olofsson, J. Org. Chem., 2010, 75, 7416-7419.54.
https://doi.org/10.1021/jo101227j
Recent Literature
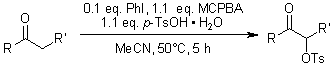
A highly efficient method was developed for synthesizing various α-tosyloxy ketones in excellent yields through the reaction of ketones with m-chloroperbenzoic acid (mCPBA) and p-toluenesulfonic acid (TsOH), catalyzed by iodobenzene.
Y. Yamamoto, H. Togo, Synlett, 2006, 798-800.
https://doi.org/10.1055/s-2006-933120
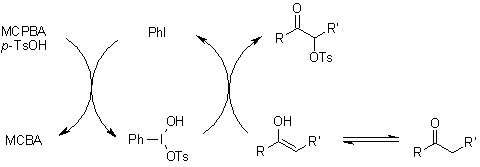
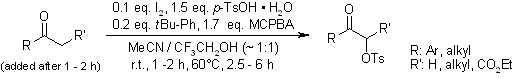
An efficient synthetic protocol enables the conversion of various ketones to α-tosyloxy ketones using m-chloroperbenzoic acid (mCPBA), p-toluenesulfonic acid (TsOH), catalytic iodine, and tert-butylbenzene in an acetonitrile/2,2,2-trifluoroethanol solvent system. The reaction proceeds via initial formation of 4-tert-butyl-1-iodobenzene, which subsequently transforms into the active α-tosyloxylation reagent 4-tert-butyl-1-[(hydroxy)(tosyloxy)iodo]benzene through reaction with mCPBA and TsOH•H₂O.
A. Tanaka, K. Moriyama, H. Togo, Synlett, 2011, 1853-1854.
https://doi.org/10.1055/s-0030-1260948
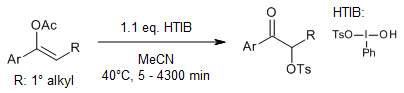
An efficient synthetic protocol enables the conversion of various ketones to α-tosyloxy ketones using m-chloroperbenzoic acid (mCPBA), p-toluenesulfonic acid (TsOH), catalytic iodine, and tert-butylbenzene in an acetonitrile/2,2,2-trifluoroethanol solvent system. The reaction proceeds via initial formation of 4-tert-butyl-1-iodobenzene, which subsequently transforms into the active α-tosyloxylation reagent 4-tert-butyl-1-[(hydroxy)(tosyloxy)iodo]benzene through reaction with mCPBA and TsOH•H₂O.
A. Tanaka, K. Moriyama, H. Togo, Synlett, 2011, 1853-1854.
https://doi.org/10.1055/s-0030-1260948
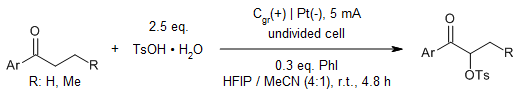
Using a simple catalytic electrosynthetic protocol, an in situ generated hypervalent iodine species eliminates chemical oxidants and the inevitable chemical waste associated with their mode of action. The developed method has been used for syntheses of dihydrooxazole and dihydro-1,3-oxazine derivatives, and the α-tosyloxylation of ketones.
M. Elsherbini, W. J. Moran, J. Org. Chem., 2023, 88, 1424-1433.
https://doi.org/10.1021/acs.joc.2c02309
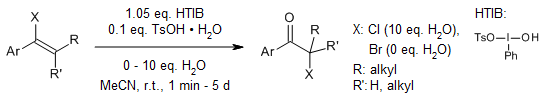
Hydroxy(tosyloxy)iodobenzene (HTIB) facilitates the oxidative rearrangement of vinyl halides to yield α-halo ketones, versatile synthetic intermediates. Mechanistic studies and enantioselective transformations are also described.
A. Jobin-Des Lauriers, C. Y. Legault, Org. Lett., 2016, 18, 108-111.
https://doi.org/10.1021/acs.orglett.5b03345
The synergistic combination of PhI(OAc)₂ and BF₃·Et₂O effectively promotes the Beckmann rearrangement of ketoximes to yield amides. This transformation involves in situ acetylation of the ketoxime hydroxyl group, which subsequently facilitates the rearrangement process. Additionally, treatment of ketoximes with Koser's reagent in THF at ambient temperature affords ketones in excellent yields.
T. Maegawa, R. Oishi, A. Maekawa, K. Segi, H. Hamamoto, A. Nakamura, Y. Miki, Synthesis, 2022, 54, 4095-4103.
https://doi.org/10.1055/a-1835-2188
A hypervalent iodine-mediated α-alkylative umpolung reaction enables efficient alkylation of carbonyl compounds using dialkylzinc reagents. This versatile method demonstrates broad applicability to various ketones, including 1,3-dicarbonyl compounds and regular ketones through their lithium enolate intermediates, delivering α-alkylated carbonyl products in excellent yields. Comprehensive mechanistic investigations involving NMR spectroscopy, trapping experiments, crossover studies, and computational analyses support an ionic reaction pathway.
O. S. Shneider, E. Pisarevsky, P. Fristrup, A. M. Szpilman, Org. Lett., 2015, 17, 282-285.
https://doi.org/10.1055/a-1835-2188
A hypervalent iodine(III) reagent enables efficient dehydrosulfurization for the straightforward synthesis of both symmetrical and unsymmetrical carbodiimides from thiourea derivatives. This oxidative transformation delivers carbodiimides in excellent yields with high selectivity. A plausible reaction mechanism has been proposed.
C. Zhu, D. Xu, Y. Wei, Synthesis, 2011, 711-714.
https://doi.org/10.1055/s-0030-1258414
The polymeric reagent poly{[4-(hydroxy)(tosyloxy)iodo]styrene} demonstrated high efficacy in the halotosyloxylation of alkynes when paired with iodine, N-bromosuccinimide (NBS), or N-chlorosuccinimide (NCS). Notably, this polymer-supported reagent exhibited excellent recyclability and reusability.
J.-M. Chen, X. Huang, Synthesis, 2004, 1557-1558.
https://doi.org/10.1055/s-2004-822373
A convenient 1,3-dipolar cycloaddition protocol utilizes in situ generated nitrile oxides from aldoximes with hydroxy(tosyloxy)iodobenzene (HTIB), reacting smoothly with maleimides, styrene, and acrylonitrile to afford isoxazoline derivatives. This methodology is particularly advantageous due to its operational simplicity, broad substrate compatibility, and absence of requirement for bases, metals, or additional additives.
R. Budhwan, M. Rawat, R. K. Peddinti, Synthesis, 2023, 55, 1904-1908.
https://doi.org/10.1055/s-0042-1752403
Using a simple catalytic electrosynthetic protocol, an in situ generated hypervalent iodine species eliminates chemical oxidants and the inevitable chemical waste associated with their mode of action. The developed method has been used for syntheses of dihydrooxazole and dihydro-1,3-oxazine derivatives, and the α-tosyloxylation of ketones.
M. Elsherbini, W. J. Moran, J. Org. Chem., 2023, 88, 1424-1433.
https://doi.org/10.1021/acs.joc.2c02309
Koser's Reagent facilitates an efficient and mild synthetic route to 3-tosyloxy-4-hydroxycoumarins, demonstrating excellent functional group compatibility.
B. Xu, Y. Gao, J. Han, Z. Xing, S. Zhao, Z. Zhang, R. Ren, L. Wang, J. Org. Chem., 2019, 84, 10136-10144.
https://doi.org/10.1021/acs.joc.9b01323
Quoted from:
https://www.organic-chemistry.org/chemicals/oxidations/kosers-reagent.shtm
Aladdin:https://www.aladdinsci.com







