Introduction
Bioorthogonal chemistry is a set of reactions that can take place in biological environments without affecting biomolecules or interfering with biochemical processes. For this purpose, the reaction must meet the following requirements: fast, efficient, and specific.
• pH: The reaction must occur at the temperatures and pH of physiological environments.
• Efficient: The reaction must provide products selectively and in high yields and must not be affected by water or endogenous nucleophiles, electrophiles, reductants, or oxidants found in complex biological environments.
• Fast: the reaction must be fast, even at low concentrations, and must form stable reaction products.
• Specific: The reaction should involve functional groups not naturally present in biological systems
History
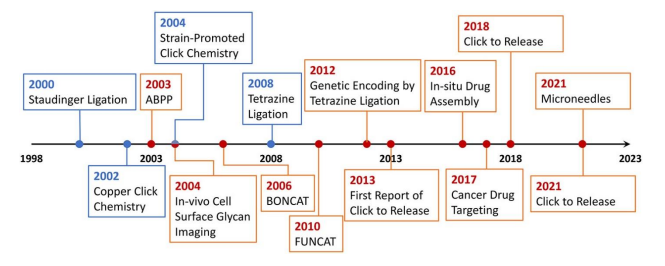
Rate constants comparisons
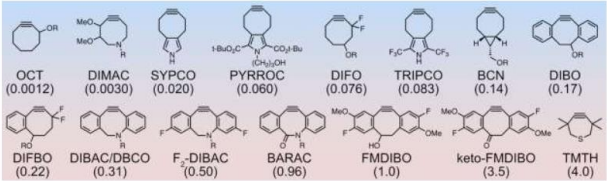
Figure 1. Chemical structures of various cyclooctyne derivatives in order of reactivity toward azides, rate constants (k2, M1
·s1
) for each of the derivatives are given in parentheses.
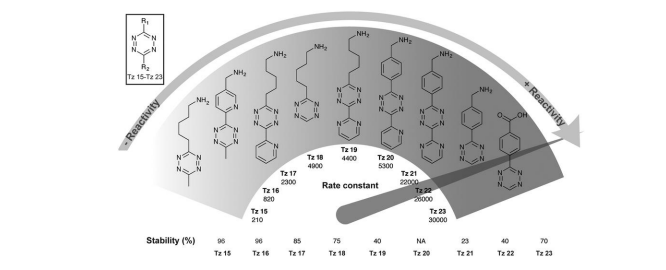
Fig. 2 Second order rate constants of selected tetrazines with TCO in PBS at 37 °C and corresponding stability assessed in PBS at 37 °C for 10 h. NA, not assessed
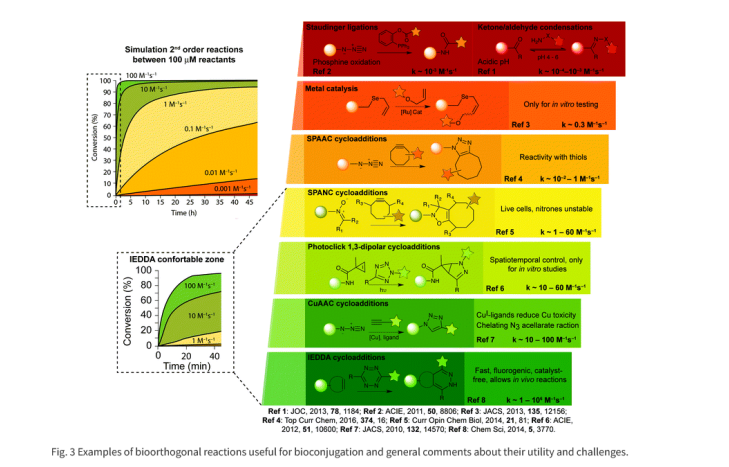
Strengths and weaknesses of bioorthogonal reactions
Table 1. Summary of Strengths and Weaknesses of Bioorthogonal Reactions
|
• CuAAC: Copper-Catalyzed Azide–Alkyne Cycloaddition;
• SPAAC: strain-promoted azide–alkyne cycloaddition;
• IEDDA: inverse electron demand Diels–Alder reaction
