Evans Blue staining is widely used in in vivo experiments in small animals to study diseases of the cardiovascular system, nervous system, and skeletal muscle system, etc. Here we focus on Evans Blue staining in combination with TTC (2, 3, 5-triphenyltetrazolium chloride) to differentiate between the danger zone and infarct zone after ischemia/reperfusion injury in the heart of mice.
Principle
By constructing a cardiac ischemia/reperfusion injury model (I/R model) in mice, the damaged mouse heart can be divided into the following three regions: (i) non-ischemic region; (ii) area at risk (AAR region); and (iii) infarcted region (Infarct size, IS region).
Evans Blue is a commonly used experimental method to observe the infarcted and undamaged regions of the myocardium. It is a water-soluble fluorescent dye, which can enter into the damaged cardiomyocytes and bind to the cell membrane to form a blue color, so the main coloring area of Evans Blue is the non-ischemic region, which is blue in color.
TTC is a redox indicator, which can be reduced to red by enzymes in cardiomyocytes, and can be used to observe the integrity of cardiomyocytes, and only cardiomyocytes with strong metabolic activity will appear red. Therefore, the main coloring area is the infarcted area, which is white, while the dangerous area will not be stained and shows the original myocardial color, which is red.
Appliance
Evans Blue + TTC double staining is a combination technique that allows simultaneous observation of cardiomyocyte integrity and metabolic activity. Cardiac Evans Blue + TTC double staining is primarily used to assess the relative sizes of the area of the danger zone and infarct zone in a mouse model of cardiac ischemia/reperfusion injury, to visualize the extent of damage after cardiac injury, and thus to localize and analyze the area of myocardial infarction.
Operation method
Heart Evans Blue / TTC Double Stain
Principle
By constructing a mouse ischemia/reperfusion injury model (I/R model), the damaged heart of mice can be divided into the following three zones: (i) non-ischemic zone, (ii) area at risk (AAR), and (iii) infarct size (IS).Evans Blue is a commonly used experimental method to observe the infarct size and undamaged size of myocardium. Evans Blue is a commonly used experimental method to observe infarcted and undamaged regions of myocardium. Evans Blue is a water-soluble fluorescent dye, which is able to enter the damaged cardiomyocytes and bind to the cell membrane to form a blue color, therefore, Evans Blue mainly colors the non-ischemic regions in blue color. Only cardiomyocytes with strong metabolic activity will show red color. Therefore, the main coloring area is the infarcted area, which is white, while the dangerous area is not stained and shows the original myocardial color, which is red.
Materials and Instruments
Small animals (mice, rats, etc.) for which I/R models have been constructed Move 1. Prepare 1.5% Evans Blue dye (1.5 g + 100 mL of distilled water) and 1% TTC dye.
1.5% Evans Blue dye, 1% TTC dye
Anesthetic, razor blade, 1 mL syringe, ophthalmic scissors
Forceps, 7-0 suture needle with thread, hemostatic forceps
Optional: small animal ventilator
2. Anesthetize the mice with anesthetics (anesthetics are not recommended here; choose the appropriate dose according to the weight of the mice).
3: Open the chest of the mouse, and quickly ligate the anterior descending vessels of the mouse heart with a 7-0 suture needle (with the assistance of a small animal ventilator if possible).
4. The ascending aorta is carefully clamped with hemostatic forceps, and Evans Blue dye (about 0.2 mL in 25 g mice) is injected slowly and carefully from the proximal end of the clamped area, and the heart of the mouse is observed to turn blue rapidly, while changes below the ligation line are not obvious.
5. Separate the hearts quickly and store them in a refrigerator at -20 or -80 ℃.
6、After collecting all the hearts according to the above steps, take out the hearts from the refrigerator and slice them layer by layer from the apical part of the heart with a razor blade, each slice is about 1 mm thick (for mice), and 5~6 slices are cut.
7. Place the slices of heart in TTC staining solution for about 10-20 minutes, and then take pictures of the slices under the in vitro microscope.
8. Statistical quantitative analysis was performed on ImaginJ software.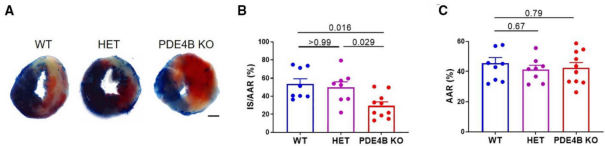
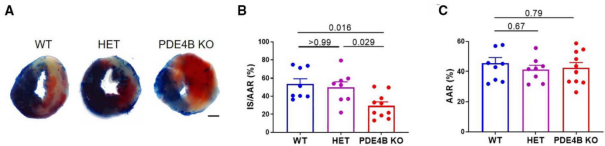
Circ Res. 2022 Aug 19;131(5):442-455. doi: 10.1161/CIRCRESAHA.122.321365. Epub 2022 Jul 28.
In Figure A, the non-ischemic areas of the mouse heart sections are blue, the infarcted areas are white, and the danger areas will not be stained and show the original myocardial color, which is red. Figure B shows the percentage of the infarcted area in the danger zone for each group. Figure C shows the percentage of the danger zone in each group.
Caveat
1. Evans Blue dye and TTC dye are best used when the dosage is calculated in advance.
2. Mice should not be over anesthetized to prevent cardiac arrest.
3. The anterior descending branch should be ligated as soon as possible after the chest is opened, and the ligation position should be the same as that in the construction of the model. Common Problems 1. What if there is no small animal ventilator? The experiment does not depend on a small animal ventilator, and it is better if there is one to prolong the beating time of the mice after the chest is opened. The experiment can be done without a small animal ventilator, but it is necessary to complete the anterior descending branch ligation, aortic clamping, and the injection of Evans Blue dye as soon as possible after the chest is opened. This is due to the lack of aortic clamping or incomplete aortic clamping, a large amount of Evans Blue will pass through the aorta into the liver and gastrointestinal tract, but hardly enter the coronary arteries, at this time, you should timely and completely clamp the aorta before the next step of dye injection. For more product details, please visit Aladdin Scientific website.
2. During the injection of Evans Blue, what is the situation that the heart does not turn blue but the liver and gastrointestinal tract do?
