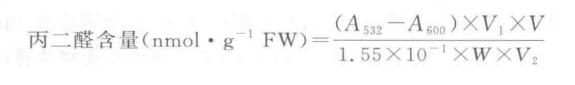To understand the factors of malondialdehyde (MDA ) formation in living organisms; to focus on the principles and methods of MDA determination; to further familiarize and master the use and precautions of spectrophotometer and centrifuge.
Principle
Malondialdehyde (MDA) is one of the end products of membrane lipid peroxidation decomposition, and its content can reflect the degree of membrane lipid peroxidation. At the same time, the accumulation of MDA in the organism will cause further damage to the cell membrane, so the content of MDA can reflect the degree of aging and suffering from adversity damage.
MDA in plant tissues heated under acidic conditions can produce a chromogenic reaction with thiobarbituric acid (TBA), and the reaction product is pink 3,5,5-trimethyloxatrile 2,4-dione. The substance has an absorption peak at 532 nm. Since thiobarbituric acid can also react with other substances and have absorption at this wavelength, in order to eliminate the effect of the reaction of thiobarbituric acid with other substances. To eliminate the effect of the reaction of thiobarbituric acid with other substances, the absorbance at 600 nm was measured at the same time as the determination of malondialdehyde, and the difference between the absorbance at 532 nm and 600 nm was used to calculate the content of malondialdehyde.
Operation method
Determination of malondialdehyde in plant tissue species
Principle
Malondialdehyde (MDA) is one of the end products of membrane lipid peroxidation decomposition, and its content can reflect the degree of membrane lipid peroxidation. At the same time, the accumulation of MDA in the organism will cause further damage to the cell membrane, so the content of MDA can reflect the degree of aging and suffering from adversity damage. MDA in plant tissues heated under acidic conditions can produce a chromogenic reaction with thiobarbituric acid (TBA), and the reaction product is pink 3,5,5-trimethyloxatrile 2,4-dione. The substance has an absorption peak at 532 nm. Since thiobarbituric acid can also react with other substances and have absorption at this wavelength, in order to eliminate the effect of the reaction of thiobarbituric acid with other substances. To eliminate the effect of the reaction of thiobarbituric acid with other substances, the absorbance at 600 nm was measured at the same time as the determination of malondialdehyde, and the difference between the absorbance at 532 nm and 600 nm was used to calculate the content of malondialdehyde.
Materials and Instruments
Material: four spinach samples, i.e. green and yellow leaves from room temperature control treatment, green and yellow leaves from high temperature treatment. Move The basic procedure for the determination of malondialdehyde content in plant tissue species can be divided into the following steps: 1. Extraction of malondialdehyde: Take 5 g of sample, add 2 mL of pre-cooled 0.05 mol - L-1 pH 7.8 phosphate buffer, add a small amount of quartz sand, grind it into a homogenate in a mortar that has been subjected to an ice bath, and transfer it to a 5 mL graduated centrifugal tube, then wash the mortar with the buffer. The washing solution was transferred into the centrifuge tube, and finally the buffer solution was fixed to 5 mL, and then centrifuged at 4 500 r - m-1 for 10 min, and the supernatant was the malondialdehyde extraction solution. 2. Determination of malondialdehyde content: Pipette 2 mL of the extract into a graduated test tube, add 3 mL of 5% thiobarbituric acid solution, and heat it in a boiling water bath for 10 min, then cool it quickly. Centrifuge at 4500 r - min-1 for 10 min and take the supernatant at 532, 600 nm wavelength, with distilled water as a blank to adjust the transmittance of 100%, and determine the absorbance. 3 . Calculation of results: In the formula to a absorbance; V1 - total volume of reaction solution, 4 mL; V a total volume of extraction solution, 5 mL; V2 a volume of extract in the reaction solution, 2 mL; W a weight of plant sample, 0.5 g; 1. 55X10-1 - micromolar absorbance coefficient of malondialdehyde (absorbance at 1 μmol of malondialdehyde in 1 L of solution). Caveat 1. 1%~0.5% TCA is more suitable for the MDA-TBA reaction, if the concentration is higher than this, the non-specific absorption of the reaction solution is high. 2. The heating time of MDA-TBA color reaction should be controlled in boiling water bath for 10~15 min, which is too short or too long to cause the decrease of light absorption at 532 nm. 3. If MDA is used as a plant senescence index, the first test should be whether the extract of the material under test can react with TBA to form an absorption peak at 532 nm. Otherwise, if only two A values at 600 nm are measured, the calculated results are not consistent with the actual situation, and the measured high A value is an artifact. 4. In the sugary material interference conditions (such as deep aging), the increase in absorbance is no longer due to lipid peroxidation product MDA content increases, but the increase in water-soluble carbohydrates, which changes the composition of the extract, can no longer be used to calculate the MDA content of the A value of 532 nm, 600 nm two, you can determine the A value of 510,532, 560 nm with A532 - (A532 - (A532)). A510-A560)/2 value was used to represent the absorbance value of the reaction solution of malondialdehyde with TBA. For more product details, please visit Aladdin Scientific website.
Reagents: 0.05 mol - L
-1
pH 7.8 sodium phosphate buffer; quartz sand;
5% trichloroacetic acid solution: 5 g of trichloroacetic acid was weighed, first dissolved in a small amount of evaporated water and then fixed to 100 mL;
0.5% thiobarbituric acid solution: weigh 0.5 g thiobarbituric acid, dissolve with 5% trichloroacetic acid, and then volume to 100 mL.
Equipment: spectrophotometer, centrifuge, water bath, balance, mortar, scissors, 5 mL graduated centrifuge tubes, 10 mL graduated test tubes, and a water bath.
10 mL graduated test tube, shackle, 5 mL, 2 mL, 1 mL pipette, refrigerator.