Introduction
Tris(3-hydroxypropyltriazolylmethyl)amine (THPTA) is a water-soluble ligand, is widely used to promote Cu-catalyzed alkyneazide cyclization (CuAAC), the typical bioorthogonal reaction with non-interference in living systems.
From mechanism, it is the copper(I) THPTA complex which accelerates the CuAAC process. CuAAC using THPTA is applicable to not only the modification of hydrophilic polymers and the bioconjugation of peptides containing an azido group, but also the rapid labeling of metabolically-incorporated azido or alkyne tags on living cell surfaces.
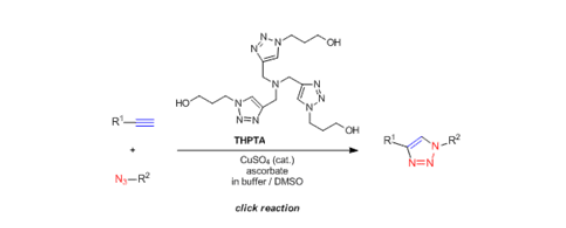
Table1. Reaction rate comparison in oligo synthesis
|
Sample protocol
1. Prepare the following click solutions
• 0.2M THPTA ligand in water
• 0.1M CuSO4 in water
• Alkyne labeled oligo in water
• 0.1M sodium ascorbate in water
• 10mM azide in DMSO/tBuOH or water
2. Pre-chelate the CuSO4 with THPTA ligand in a 1:1 ratio several minutes before the reaction. This solution is stable for several weeks when frozen.
3. To the oligo solution, add an excess of azide (4-50 eq).
4. Add 25 equivalents of THPTA/CuSO4.
5. Add 40 equivalents of sodium ascorbate.
6. The solution can be degassed briefly with an inert gas.
7. Let the reaction stand at room temperature for 15-60 minutes.
8. Ethanol-precipitate the oligo or purify using Glen Gel-Pak.
References
1. Polytriazoles as Copper(I)-Stabilizing Ligands in Catalysis T. R. Chan, R. Hilgraf, K. B. Sharpless, V. V. Fokin, Org. Lett. 2004, 6, 2858.
2. Analysis and Optimization of Copper-Catalyzed Azide–Alkyne Cycloaddition for Bioconjugation V. Hong, S. I. Presolski, C. Ma, M. G. Finn, Angew. Chem. Int. Ed. 2009, 48, 9879.
3. Stabilization of Virus-like Particles with Poly(2-oxazoline)s F. Manzenrieder, R. Luxenhofer, M. Retzlaff, R. Jordan, M. G. Finn, Angew. Chem. Int. Ed. 2011, 50, 2601.
4. Synthesis of Cyclic Peptide Disulfide–PHPMA Conjugates via Sequential Active Ester Aminolysis and CuAAC Coupling; K. A. Günay, H. Klok, Polym. Chem. 2016, 7, 970.
5. a) Cellular Consequences of Copper Complexes used to Catalyze Bioorthogonal Click Reactions D. C. Kennedy, C. S. McKay, M. C. B. Legault, D. C. Danielson, J. A. Blake, A. F. Pegoraro, A. Stolow, Z. Mester, J. P. Pezacki, J. Am. Chem. Soc. 2011, 133, 17993.
b) Labeling Live Cells by Copper-Catalyzed Alkyne−Azide Click Chemistry;V. Hong, N. F. Steinmetz, M. Manchester,
M. G. Finn, Bioconjugate Chem. 2010, 21, 1912.
