Sandra Forbes
Product Manager

Solvothermal synthesis represents a versatile technique for the preparation of diverse materials encompassing metals, semiconductors, ceramics, and polymers. This process entails the utilization of a solvent under conditions of moderate to high pressure (ranging typically from 1 atm to 10,000 atm) and temperature (commonly between 100 °C and 1000 °C), which promotes the interaction of precursors during the synthesis phase. When water serves as the solvent, the process is designated as "hydrothermal synthesis." Typically, synthesis under hydrothermal conditions occurs below the supercritical temperature of water, which is 374 °C. This methodology can produce various geometric forms, including thin films, bulk powders, single crystals, and nanocrystals. Furthermore, the morphology of the resulting crystals, whether spherical (3D), rod-shaped (2D), or wire-like (1D), can be controlled by adjusting factors such as solvent supersaturation, the concentration of the target chemical, and kinetic control. This technique is capable of preparing both thermodynamically stable and metastable states, including novel materials that are difficult to synthesize through alternative routes. In recent years, approximately 80% of the literature on solvothermal synthesis has concentrated on nanocrystals; consequently, this review will emphasize some of the advancements in nanocrystalline solvothermal synthesis.
The fascination with nanocrystals stems from their exceptional properties, exemplified by the discovery of solvothermally synthesized quantum dots (QDs). Luis Brus1,2 initially demonstrated that hydrothermally prepared cadmium sulfide (CdS) nanoparticles suspended in water exhibited a blue shift in their visible absorption and emission spectra compared to bulk CdS. Nanoparticles with radii smaller than the exciton Bohr radius display discrete energy levels akin to individual atoms. Unlike the continuous band energies seen in bulk materials, each unique nanoscale crystal diameter corresponds to a specific discrete energy level. Materials displaying this trait are termed "artificial atoms" or quantum dots. Recent reviews3-5 underscore the significance of solvothermal synthesis as a crucial technique for tailoring the size of II–VI and III–V semiconductor materials. Typically, synthesizing these QDs necessitates a soluble cation source material in the chosen solvent and a surfactant to cap or stabilize the quantum dot, thereby halting its growth. For instance, CdSe QDs are synthesized by dissolving CdO in trioctylphosphine oxide (TOPO) and trioctylphosphine (TOP), which serve dual roles as solvent and capping agent. The solution is heated to 300 °C, followed by the addition of elemental selenium dissolved in tributylphosphine (TBP). The reaction is then rapidly cooled, resulting in the formation of nanocrystals.6
Zinc oxide serves as another illustration of a II–VI compound that can be synthesized through solvothermal methods and demonstrates quantum dot behavior.7 In a particular synthesis approach, zinc acetate dihydrate was dissolved in 2-propanol at a temperature of 50 °C. Following this, the solution was cooled down to 0 °C, and NaOH was introduced to precipitate ZnO. The mixture was then heated up to 65 °C to facilitate ZnO growth over a specific duration. Afterward, a capping agent, namely 1-dodecanethiol, was injected into the suspension to halt the growth process. The resultant rod-shaped Zinc oxide nanocrystals (depicted in Figure 1) exhibit an absorption spectrum (displayed in Figure 2) that is indicative of the quantum effect.
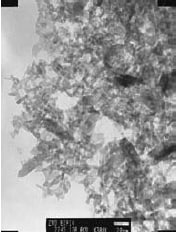
Figure 1. TEM micrograph of ZnO rods

Figure 2. Absorption plot of ZnO rods showing quantum dot effect
Quantum dots can be synthesized in various shapes such as spheres, rods, tetrapods, and teardrops through the solvothermal method by precisely managing the temperature, concentration, and duration of the reaction.8 Furthermore, it is possible to encapsulate a core nanocrystal (for example, cadmium sulfide)9 with a shell of a different composition (such as ZnS). The core can serve as a seed for growing larger particles by modifying the concentration after the initial growth stage. Size and shape control are crucial for optimizing numerous quantum dot applications, and solvothermal synthesis stands as a pivotal technology in achieving this precision.
In comparison to II–VI materials, III–V compounds pose greater challenges for processing via the solvothermal method.4 One technique for synthesizing nanosized InSb involves the reduction of InCl3 and SbCl3 using NaBH4 at a temperature of 200 °C, with diethylenediamine (DETA) serving as the solvent.10 The resulting material, however, exhibited irregular shapes and agglomeration, preventing it from displaying the quantum dot effect. Conversely, recent research on III–V semiconductors such as phosphide (InP), nitride (GaN), and arsenide (GaAs) has demonstrated quantum dot properties.
While metallic particles can indeed exhibit quantum dot behavior, their exciton Bohr radius is considerably smaller than that of semiconductors, posing significant synthetic hurdles. However, the synthesis of metallic nanoparticles is currently of great interest for applications in nanocircuits and devices. The size, shape, and material type required depend on the specific application. For instance, the pursuit of higher-density magnetic recording devices led to the development of a novel nano-sized ferromagnetic material based on the 3D self-assembly of two distinct sizes of magnetic particles—Fe3O4 (8 nm) and Fe58Pt42 (4 nm)—into a superlattice colloidal crystal. This self-assembly occurs when the particles have a size distribution of less than 5%. Monosized Fe3O4 particles were prepared from iron (III) acetylacetonate in phenyl ether, in the presence of alcohol, oleic acid, and oleylamine at 265 °C.11 Meanwhile, monosized Fe58Pt42 was synthesized through the reduction of platinum acetylacetonate by 1,2-hexadecanediol and the decomposition of iron pentacarbonyl, with oleic acid and oleyl amine serving as stabilizers.11
Magnetic particles, metallic nanoparticles, and quantum dots of similar types are being utilized in biosensors. These nanoparticles necessitate hydrophilic surface moieties to ensure compatibility with biomolecules. Nanoparticles prepared through the hydrothermal method are particularly advantageous for biotechnology applications because their hydrophilic nature stems from surface hydroxyl groups. However, these hydroxyl groups can often impact the nanoparticles' desired properties, such as reducing the quantum yield of QDs or oxidizing metal surfaces. Alternatively, other solvothermal methods can be employed to create nanoparticles that become hydrophilic upon the addition of surfactants. Gold particles are of particular interest due to their inert nature. Monosized gold particles were synthesized using a solvothermal reduction process akin to that described by Chen and Kimura.12 In this technique, hydrogen tetrachloroaurate(III) was reduced with sodium borohydride, while mercaptosuccinic acid served as a stabilizer. Figure 3 depicts a micrograph of gold nanoparticles self-assembled onto a copper surface.
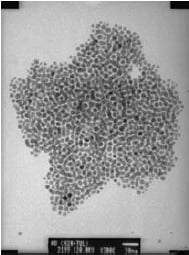
Figure 3. TEM micrograph of self-assembled gold particles
In summary, the solvothermal synthesis of nanoparticles is gaining widespread application across various fields, including nanocircuits, nano-optical circuits, nanomagnetics, and biotechnology. This technique's versatility, economy, and simplicity stem from its ability to control the size and shape of a diverse range of materials.
References
1. Rossetti R, Brus L. 1982. Electron-hole recombination emission as a probe of surface chemistry in aqueous cadmium sulfide colloids. J. Phys. Chem.. 86(23):4470-4472. https://doi.org/10.1021/j100220a003
2. Brus LE. 1984. Electron-electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. The Journal of Chemical Physics. 80(9):4403-4409. https://doi.org/10.1063/1.447218
3. Esteves ACC, Trindade T. 2002. Synthetic studies on II/VI semiconductor quantum dots. Current Opinion in Solid State and Materials Science. 6(4):347-353. https://doi.org/10.1016/s1359-0286(02)00079-7
4. Green M. 2002. Solution routes to III-V semiconductor quantum dots. Current Opinion in Solid State and Materials Science. 6(4):355-363. https://doi.org/10.1016/s1359-0286(02)00028-1
5. Rajamathi M, Seshadri R. 2002. Oxide and chalcogenide nanoparticles from hydrothermal/solvothermal reactions. Current Opinion in Solid State and Materials Science. 6(4):337-345. https://doi.org/10.1016/s1359-0286(02)00029-3
6. Peng Q, Dong Y, Deng Z, Sun X, Li Y. 2001. Low-Temperature Elemental-Direct-Reaction Route to II-VI Semiconductor Nanocrystalline ZnSe and CdSe. Inorg. Chem.. 40(16):3840-3841. https://doi.org/10.1021/ic0100424
7. Wong EM, Hoertz PG, Liang CJ, Shi B, Meyer GJ, Searson PC. 2001. Influence of Organic Capping Ligands on the Growth Kinetics of ZnO Nanoparticles. Langmuir. 17(26):8362-8367. https://doi.org/10.1021/la010944h
8. Manna L, Scher EC, Alivisatos AP. 2000. Synthesis of Soluble and Processable Rod-, Arrow-, Teardrop-, and Tetrapod-Shaped CdSe Nanocrystals. J. Am. Chem. Soc.. 122(51):12700-12706. https://doi.org/10.1021/ja003055+
9. Manna L, Scher EC, Li L, Alivisatos AP. 2002. Epitaxial Growth and Photochemical Annealing of Graded CdS/ZnS Shells on Colloidal CdSe Nanorods. J. Am. Chem. Soc.. 124(24):7136-7145. https://doi.org/10.1021/ja025946i
10. Lezaeta MD, Lam M, Black S, Chang B, Gersten B. 2004. The Synthesis of III-V Semiconductor InSb Nanoparticles by Solvothermal Reduction Reactions. MRS Proc.. 848 https://doi.org/10.1557/proc-848-ff3.34
11. Zeng H, Li J, Liu JP, Wang ZL, Sun S. 2002. Exchange-coupled nanocomposite magnets by nanoparticle self-assembly. Nature. 420(6914):395-398. https://doi.org/10.1038/nature01208
12. Chen S, Kimura K. 1999. Synthesis and Characterization of Carboxylate-Modified Gold Nanoparticle Powders Dispersible in Water. Langmuir. 15(4):1075-1082. https://doi.org/10.1021/la9812828
Aladdinsci: https://www.aladdinsci.com
