Product Manager:Nick Wilde
Introduction
1-Hydrosilatrane, depicted in Figure 1, serves as a highly versatile hydride reagent. It can be employed in various reduction reactions to synthesize alcohols, amines, their chiral derivatives, and esters from aldehydes and ketones.1-6 Its relative reactivity compared to other silanes, coupled with its moisture stability and ease of handling, renders it an appealing choice as a reducing agent.
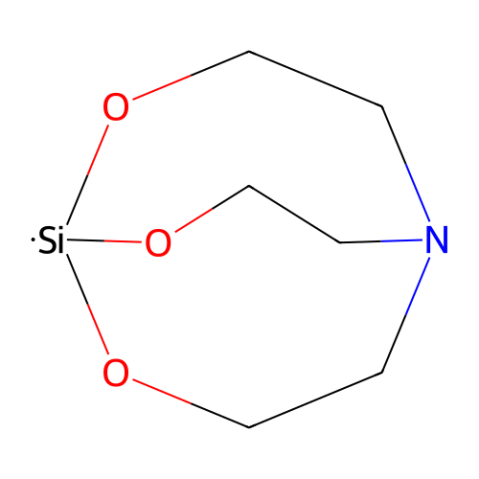
Figure 1. 1-Hydrosilatrane.
Frye et al. reported silatranes in 1961 as the pioneering examples of stable pentacoordinate alkoxysilanes.7 A key characteristic of silatranes lies in their caged structure, which promotes the formation of a dative bond between nitrogen and silicon.7-9 Hypercoordinate organosilanes exhibit enhanced electropositivity at the silicon center, leading to an increase in electron density at the ligands.10 Despite their numerous beneficial properties, silatranes have not gained widespread use as reagents in organic synthesis.11
In 1976, Eaborn and his team were the pioneers in exploring 1-hydrosilatrane as a source of hydride, presenting an unoptimized example of its use in reducing six distinct functional groups.12 Hydrosilanes function as hydride sources due to the polarization of the Si-H bond towards hydrogen.13 The intramolecular coordination between nitrogen and silicon in 1-hydrosilatrane enhances the reactivity of the Si-H bond, making it a potent hydride donor.8,9 Despite its high stability attributed to its rigid structure, initial work only provided preliminary insights into 1-hydrosilatrane's potential as a reducing agent. It was not until recently, starting in 2016, that the Adler group reported efficient synthetic methods utilizing 1-hydrosilatrane.1-6
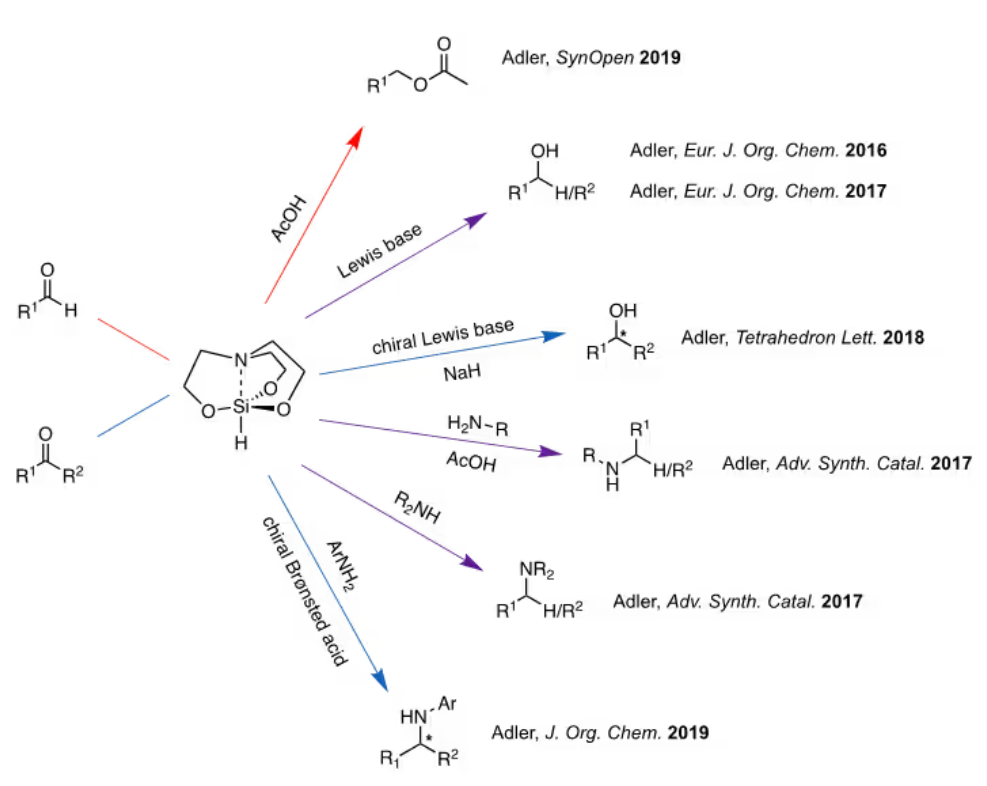
Figure 2. Methods utilizing 1-hydrosilatrane as a hydride reducing reagent
Benefits of 1-Hydrosilatrane Use
1-Hydrosilatrane serves as a stable reducing agent in the presence of air and moisture, suitable for use in open-air benchtop reactions. It is non-toxic, user-friendly, and maintains its integrity over time.2 As a safer alternative to metal hydride reductions, it offers straightforward methods that operate under gentle conditions. This reducing agent is compatible with numerous functional groups, exhibits chemoselectivity, and generates non-toxic byproducts. Moreover, it is scalable, can be used without metals, and is capable of synthesizing biologically significant molecules and pharmaceuticals.
Representative Applications
1. Direct Reductive Amination
Direct reductive amination (DRA) is the most frequently employed technique for synthesizing secondary and tertiary amines.14 Effective DRA methods require a chemoselective hydride source to reduce the imine or iminium intermediate without affecting the initial carbonyl group or other functionalities. 1-Hydrosilatrane, a highly reactive reducing agent, is well-suited for DRA due to its compatibility with various functional groups.1,2 Research has demonstrated the broad applicability of 1-hydrosilatrane in DRA reactions with aldehydes/ketones and secondary amines to produce tertiary amines (Figure 3), showcasing its excellent tolerance towards reducible and common protecting groups. Additionally, secondary amines were synthesized using aldehydes/ketones in acetic acid with 1-hydrosilatrane (Figure 3). Notably, DRA reactions with 1-hydrosilatrane exhibit chemoselectivity towards aldehydes, even in the presence of ketone substituents on the same molecule. Consequently, amino alcohols can be synthesized in a one-pot reduction process using 1-hydrosilatrane (Figure 4).3
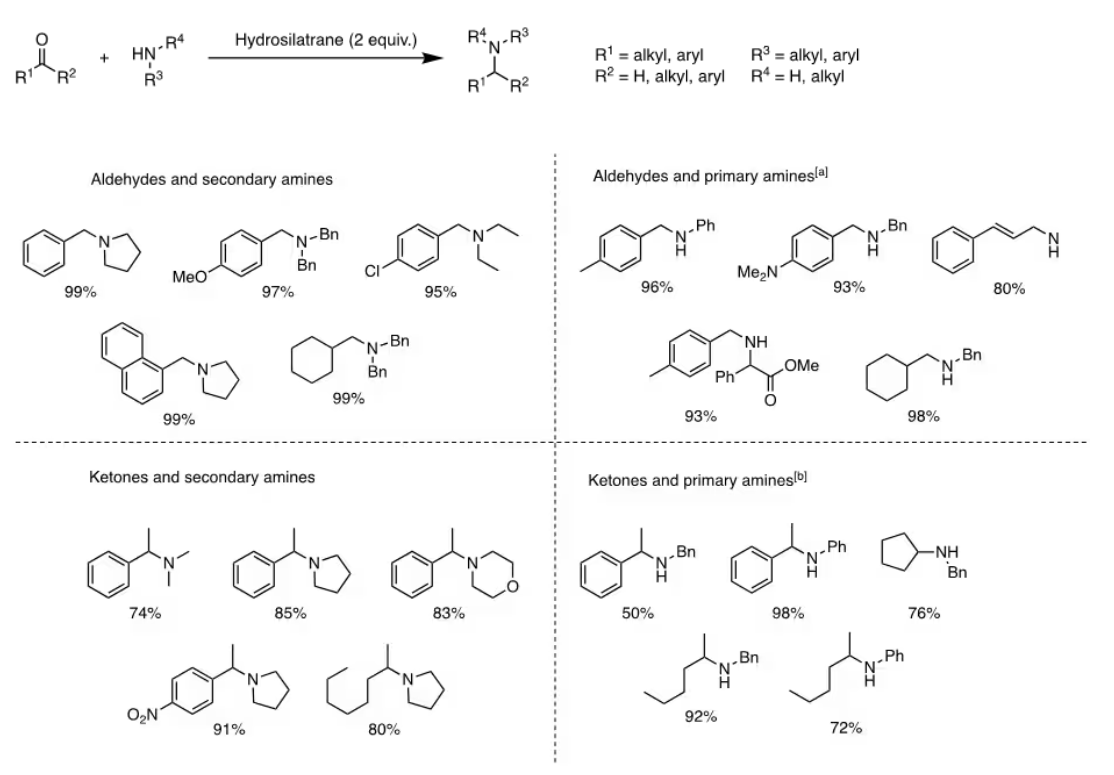
Figure 3.Direct reductive amination using 1-hydrosilatrane. [a] AcOH, r.t. [b] AcOH, 70˚C.
Figure 4.Two-step one-pot tandem DRA/reduction of a ketoaldehyde using 1-hydrosilatrane.
Many crucial amines exhibit chirality, rendering asymmetric diastereoselective reductive amination (DRA) a crucial process. To achieve enantioselectivity, a large chiral phosphoric acid catalyst can be employed in the DRA of prochiral ketones with arylamines, utilizing 1-hydrosilatrane as the reducer. This results in the production of chiral amines with high conversion rates and excellent enantiomeric excess (see Figure 5).6
Figure 5. Asymmetric DRA using 1-hydrosilatrane and a chiral phosphoric acid catalyst.
2. Reduction of Ketones
When Lewis basic tBuOK is added, 1-hydrosilatrane becomes activated and is capable of reducing a variety of ketones to their corresponding alcohols under gentle conditions, without generating volatile or hazardous silanes (Figure 6). The reduction of ketones with 1-hydrosilatrane can be achieved with high diastereoselectivity. Furthermore, the asymmetric reduction of ketones can exhibit enantioselectivity when a chiral additive is included, allowing for the synthesis of chiral alcohols with high enantiomeric excess (Figure 7).2,4
Figure 6.Reduction of ketones with 1-hydrosilatrane.
Figure 7.Asymmetric reduction of prochiral ketones with 1-hydrosilatrane and a chiral Lewis base.
3. Reduction of Aldehydes
1-Hydrosilatrane can be employed to convert aldehydes into their corresponding primary alcohols. When activated by NaOH, it effectively reduces a variety of aryl aldehydes to benzylic alcohols, without producing ethers or deoxygenated byproducts (as shown in Figure 8).1 No cross-reactivity was noted with reducible nitriles, nitro groups, or benzyl/allyl ethers. Moreover, heteroaryl, polycyclic aryl, and aliphatic aldehydes have also been efficiently reduced using 1-hydrosilatrane.1
Figure 8.Reduction of substituted benzaldehydes.
Aldehydes can directly undergo reductive acetylation to produce acylated alcohols, which is advantageous for synthesizing esters or protecting alcohols during synthesis. This process can enhance the efficiency of multistep synthetic routes. By using 1-hydrosilatrane as a reducing agent in the presence of acetic acid and an aldehyde, various acetyl esters can be synthesized in good to excellent yields (as illustrated in Figure 9). Notably, this method does not produce ether by-products. It demonstrates chemoselectivity towards aldehydes, as ketones on the same molecule remain unreduced when exploring the scope of substrates.5
Figure 9. Direct reductive acetylation of aldehydes.
References
1. Skrypai V, Hurley JJM, Adler MJ. 2016. Silatrane as a Practical and Selective Reagent for the Reduction of Aryl Aldehydes to Benzylic Alcohols. Eur. J. Org. Chem.. 2016(12):2207-2211. https://doi.org/10.1002/ejoc.201501599
2. Varjosaari SE, Skrypai V, Suating P, Hurley JJM, Gilbert TM, Adler MJ. 2017. 1-Hydrosilatrane: A Locomotive for Efficient Ketone Reductions. Eur. J. Org. Chem.. 2017(2):229-232. https://doi.org/10.1002/ejoc.201601256
3. Varjosaari SE, Skrypai V, Suating P, Hurley JJM, Lio AMD, Gilbert TM, Adler MJ. 2017. Simple Metal-Free Direct Reductive Amination Using Hydrosilatrane to Form Secondary and Tertiary Amines. Adv. Synth. Catal.. 359(11):1872-1878. https://doi.org/10.1002/adsc.201700079
4. Varjosaari SE, Skrypai V, Herlugson SM, Gilbert TM, Adler MJ. 2018. Enantioselective metal-free reduction of ketones by a user-friendly silane with a reusable chiral additive. Tetrahedron Letters. 59(29):2839-2843. https://doi.org/10.1016/j.tetlet.2018.06.032
5.James, R.; Herlugson, S.; Varjosaari, S.; Skrypai, V.; Shakeel, Z.; Gilbert, T.; Adler, M. J. SynOpen 2019, 3, 1-3.
6.Skrypai V, Varjosaari SE, Azam F, Gilbert TM, Adler MJ. 2019. Chiral Brønsted Acid-Catalyzed Metal-Free Asymmetric Direct Reductive Amination Using 1-Hydrosilatrane. J. Org. Chem.. 84(9):5021-5026. https://doi.org/10.1021/acs.joc.8b03073
7.Frve CL, Vogel GE, Hall JA. 1961. TRIPTYCH-SILOXAZOLIDINES: PENTACOORDINATE BRIDGEHEAD SILANES RESULTING FROM TRANSANNULAR INTERACTION OF NITROGEN AND SILICON. J. Am. Chem. Soc.. 83(4):996-997. https://doi.org/10.1021/ja01465a058
8.Pestunovich V, Kirpichenko S, Voronkov M. Silatranes and Their Tricyclic Analogs.1447-1537. https://doi.org/10.1002/0470857250.ch24
9.Frye CL, Vincent GA, Finzel WA. 1971. Pentacoordinate silicon compounds. V. Novel silatrane chemistry. J. Am. Chem. Soc.. 93(25):6805-6811. https://doi.org/10.1021/ja00754a017
10.Oestreich M, Rendler S. 2005. Hypervalent Silicon as a Reactive Site in Selective Bond-Forming Processes. Synthesis. 2005(11):1727-1747. https://doi.org/10.1055/s-2005-869949
11.Puri JK, Singh R, Chahal VK. Silatranes: a review on their synthesis, structure, reactivity and applications. Chem. Soc. Rev.. 40(3):1791-1840. https://doi.org/10.1039/b925899j
12.Attar-bashi, M. T.; Eaborn, C.; Vencl, J.; Walton, D. R. M. J. Organomet. Chem. 1976, 117, 87-89.
13.Doyle MP, DeBruyn DJ, Donnelly SJ, Kooistra DA, Odubela AA, West CT, Zonnebelt SM. 1974. Silane reductions in acidic media. III. Reductions of aldehydes and ketones to alcohols and alcohol derivatives. General syntheses of alcohols, symmetrical ethers, carboxylate esters and acetamides. J. Org. Chem.. 39(18):2740-2747. https://doi.org/10.1021/jo00932a015
14.Podyacheva E, Afanasyev OI, Tsygankov AA, Makarova M, Chusov D. 2019. Hitchhikers Guide to Reductive Amination. Synthesis. 51(13):2667-2677. https://doi.org/10.1055/s-0037-1611788
Aladdin:https://www.aladdinsci.com








