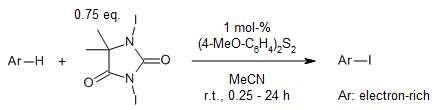Product Manager:Nick Wilde
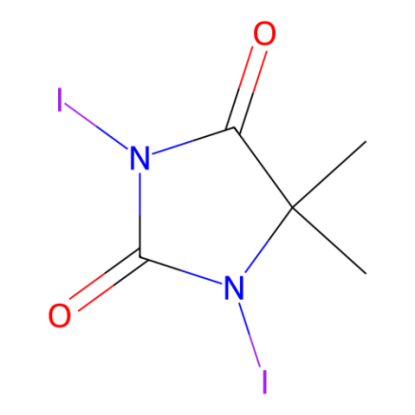
1,3-Diiodo-5,5-dimethylhydantoin(DIH) exhibits a reactivity similar to molecular iodine but is easier to manage since it does not sublime as a solid reagent. Additionally, DIH serves as a viable substitute for NIS (N-iodosuccinimide) in the iodination of arenes or enols.
Recent Literature
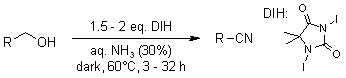
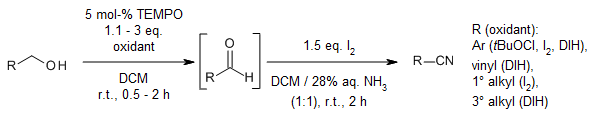
Primary alcohols, as well as primary, secondary, and tertiary amines, were successfully transformed into their respective nitriles in high yields through oxidation using 1,3-Diiodo-5,5-dimethylhydantoin(DIH) in an aqueous ammonia solution at a temperature of 60 °C.
S. Iida, H. Togo, Synlett, 2007, 407-410.
https://doi.org/10.1055/s-2007-967954
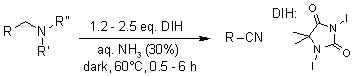
S. Iida, H. Togo, Synlett, 2007, 407-410.
https://doi.org/10.1055/s-2007-967954
At room temperature, various alcohols were effectively converted into their corresponding nitriles through treatment with tert-butyl hypochlorite, diiodine, or 1,3-Diiodo-5,5-dimethylhydantoin(DIH) in the presence of TEMPO. Subsequent treatment with diiodine and aqueous ammonia further processed the reaction. The resulting nitriles, obtained in good yields and high purities, were easily isolated by extracting the reaction mixture with chloroform and subsequently removing the solvent.
H. Shimojo, K. Moriyama, H. Togo, Synthesis, 2013, 45, 2155-2156.
https://doi.org/10.1055/s-2007-967954
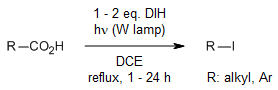
A versatile and effective method has been developed for converting both aliphatic and aromatic carboxylic acids into organic iodides, without the need for heavy metals or potent oxidizing agents. This process utilizes commercially available N-iodoamides, which serve dual roles as initiators and halogen donors under irradiation. The isolation of the product is straightforward, and the primary by-product is readily soluble in water.
K. Kulbitski, G. Nisnevich, M. Gandelman, Adv. Synth. Catal., 2011, 353, 1438-1442.
https://doi.org/10.1002/adcs.201100145
In an electrophilic iodination process utilizing 1,3-Diiodo-5,5-dimethylhydantoin(DIH) and catalyzed by a disulfide, the disulfide functions as a Lewis base to activate DIH. This activation facilitates the iodination reaction in acetonitrile under gentle conditions. The system demonstrates versatility across a broad spectrum of substrates, encompassing anisoles, acetanilides, imidazoles, and pyrazoles.
K. Iida, S. Ishida, T. Watanabe, T. Arai, J. Org. Chem., 2019, 84, 7411-7417.
https://doi.org/10.1021/acs.joc.9b00769
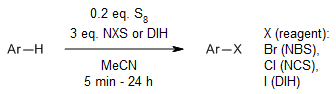
Elemental sulfur (S8) serves as a mediator in aromatic halogenation reactions, employing N-halosuccinimides for bromination and chlorination, or 1,3-Diiodo-5,5-dimethylhydantoin(DIH) for iodination. This process is effective even with less reactive aromatic compounds, including those substituted with esters, cyano groups, and nitros, such as anisole derivatives.
J. Matsuoka, Y. Yano, Y. Hirose, K. Mashiba, N. Sawada, A. Nakamura, T. Maegawa, J. Org. Chem., 2024, 89, 770-777.
https://doi.org/10.1055/s-0033-1338468
An organocatalyzed iodination of activated aromatic compounds, employing 1,3-Diiodo-5,5-dimethylhydantoin(DIH) as the iodine source and thiourea catalysts in acetonitrile, is applicable to various aromatic substrates with diverse steric and electronic properties. This process typically exhibits high regioselectivity and yields substantial quantities of isolated products.
G. Jakab, A. Hosseini, H. Hausmann, P. R. Schreiner, Synthesis, 2013, 45, 1635-1640.
https://doi.org/10.1055/s-0033-1338468
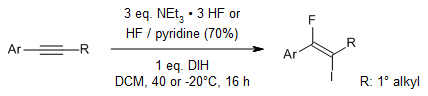
Utilizing 1,3-Diiodo-5,5-dimethylhydantoin(DIH) alongside HF-based reagents allows for a regio- and stereoselective iodofluorination of both internal and terminal alkynes. Additionally, a straightforward approach for achieving controlled regioselective double iodofluorination of terminal alkynes is introduced.
L. Pfeifer, V. Gouverneur, Org. Lett., 2018, 20, 1576-1579.
https://doi.org/10.1021/acs.orglett.8b00321
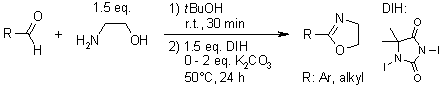
A range of aromatic and aliphatic aldehydes were efficiently transformed into their corresponding 2-aryl and 2-alkyl-2-oxazolines, respectively, in good yields through reaction with 2-aminoethanol and 1,3-Diiodo-5,5-dimethylhydantoin(DIH).
S. Takahashi, H. Togo, Synthesis, 2009, 2329-2332.
https://doi.org/10.1055/s-0029-1216843
Quoted
from:https://www.organic-chemistry.org/chemicals/oxidations/dih-1,3-diiodo-5,5-dimethylhydantoin.shtm
Aladdin:https://www.aladdinsci.com