Sandra Forbes
Product Manager

Ammonia borane/Borane ammonia complex white solid can be used as an air-stable alternative to diborane.
![]()
Recent Literature
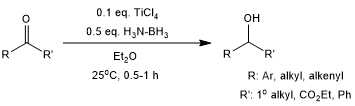
A catalytic amount of titanium tetrachloride immensely accelerates the hydroboration-hydrolysis (reduction) of ketones with ammonia borane in diethyl ether at room temperature. The product alcohols are produced in very good yields within 30 min, even with ketones which typically requires 24 h or longer under uncatalyzed conditions.
P. V. Ramachandran, A. A. Alawaed, H. J. Hamann, J. Org. Chem., 2022, 87, 13259-13269. https://doi.org/10.1021/acs.joc.2c01744
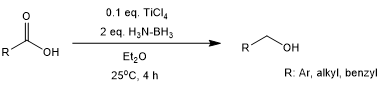
Ammonia-borane reduces acids to alcohols at room temperature in the presence of catalytic TiCl4. This process tolerates a variety of potentially reactive functional groups, including N-protected amino acids, nitriles and, to some extent, esters. Aliphatic acids can be selectively reduced in the presence of aromatic acids.
P. V. Ramachandran, A. A. Alawaed, H. J. Hamann, Org. Lett., 2022, 24, 8481-8486. https://doi.org/10.1021/acs.orglett.2c03326
Trimethyl borate promotes a solvent-free reductive amination of aldehydes and ketones with aliphatic and aromatic amines in very good yields in the presence of ammonia borane as reductant.
P. V. Ramachandran, S. Choudhary, A. Singh, J. Org. Chem., 2021, 86, 4274-4280. https://doi.org/10.1021/acs.joc.0c02143
A wide range of nitriles were reduced to primary amines by 1.2 equiv of ammonia borane under thermal decomposition conditions without any catalyst to primary amines in very good yields. The reactions are environmentally benign with H2 and NH3 generated as byproducts. The reactions are also tolerant of many functional groups.
M. Ding, J. Chang, J.-X. Mao, J. Zhang, X. Chen, J. Org. Chem., 2022, 87, 16230-16235. https://doi.org/10.1021/acs.joc.2c01727
A cobalt-catalyzed stereodivergent transfer hydrogenation of alkynes provides either Z- or E-alkenes based on a rational catalyst design. Substrates bearing a wide range of functional groups can be hydrogenated in good yields using catalyst loadings as low as 0.2 mol %.
S. Fu, N.-Y. Chen, X. Liu, Z. Shao, S.-P. Luo, Q. Liu, J. Am. Chem. Soc., 2016, 138, 8588-8594. https://doi.org/10.1021/jacs.6b04271
Nickel catalyzes a semihydrogenation of azoarenes with NH3BH3 to provide hydrazoarenes with good functional group tolerance and a high turnover frequency at room temperature. Results of control and deuterium-labeling experiments indicate that the ethanol hydroxyl and BH3 groups each donated one hydrogen to this transfer hydrogenation, and the main byproducts were B(OEt)3 and H2.
D. Gong, D. Kong, Y. Li, C. Gao, L. Zhao, Org. Lett., 2023, 25, 4168-4172. https://doi.org/10.1021/acs.orglett.3c01529
A borane catalyzed metal-free transfer hydrogenation of pyridines furnishes various piperidines in good yields with good cis-selectivities in the presence of ammonia borane as a hydrogen source. The ease in handling without requiring high pressure H2 makes this transfer hydrogenation practical and useful.
Q. Zhou, L. Zhang, W. Meng, X. Feng, J. Yang, H. Du, Org. Lett., 2016, 18, 5189-5191. https://doi.org/10.1021/acs.orglett.6b02610
Quoted from: https://www.organic-chemistry.org/chemicals/reductions/ammonia-borane.shtm
Aladdinsci: https://www.aladdinsci.com/






