Product Manager:Nick Wilde
Abstract
Visible-light photoredox catalysis represents a significant and expanding research domain within the realm of eco-friendly and sustainable organic synthesis. Our group has conducted extensive research on the single-electron transfer (SET) mechanism in photoredox catalysis, aiming to develop more environmentally benign carbon-carbon bond formation reactions and oxaziridine rearrangements. This review focuses on our achievements in developing and utilizing neutral silicon radical precursors to produce alkyl radicals and achieve selective rearrangement of oxaziridines into nitrones or amides.
Introduction
In recent years, visible-light photoredox catalysis has garnered considerable attention in sustainable organic synthesis due to its utilization of inexpensive, abundant, and clean light sources, along with gentle reaction conditions. These advantages have facilitated the development of numerous organic reactions catalyzed by visible-light photocatalysts.1 One of the primary mechanisms underlying photoredox catalysis is single-electron transfer (SET), which can occur through oxidative or reductive quenching cycles depending on the catalyst's redox state during the catalytic process. The SET mechanism can be classified into oxidative and reductive quenching cycles according to the redox state of the catalyst in the catalytic cycle (Figure 1, Part (a)).1,2 The spontaneity of the SET mechanism can be easily predicted from the Gibbs energy of a photoinduced electron transfer (ΔGPET =-F[Ered(A/A•–)−Eox(D•+/D)]−w−ΔE0,0), where the redox potentials of catalysts and substrates can be obtained from the literature or determined through cyclic voltammetry.Our research has focused on employing SET in photoredox catalysis for carbon-carbon bond formation and oxaziridine rearrangement reactions using transition-metal-based and organic photocatalysts, such as Ru(bpz)3(PF6)2, Ir(dF(CF3)ppy)2(dtbpy)PF6, Fukuzumi's acridinium salt(Acr+-Mes), 2,4,5,6-tetrakis(9H-carbazol-9-yl)-isophthalonitrile (4CzIPN), and 2,4,5,6-tetrakis(3,6-dichloro-9H-carbazol-9-yl)isophthalonitrile (Cl-4CzIPN), which are good oxidizers (Figure 1, Part (b)). We have particularly favored organic photocatalysts due to their cost-effectiveness and straightforward preparation. This review summarizes our findings on the use of neutral silicon-based radical precursors for generating alkyl radicals through SET and enabling the selective rearrangement of oxaziridines into nitrones or amides.
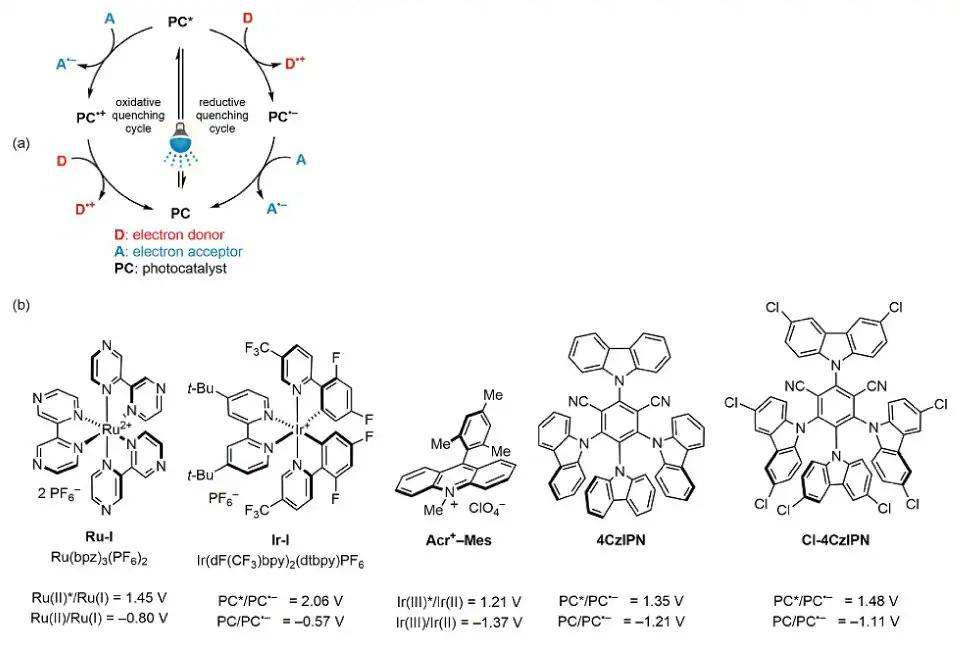
Figure 1.(a)Single-Electron Transfer (SET) Mechanism. (b)Popular Oxidizer Photocatalysts. (Ref. 1)
Conclusion
In this review, we have outlined our contributions to the advancement of environmentally friendly and sustainable reactions involving single-electron transfer (SET) mechanisms in photoredox catalysis. Specifically, we have developed neutral, silicon-based precursors for alkoxymethyl, hydroxymethyl, and allylic radicals, which we have utilized in Giese reactions, radical-polar crossover (RPC) reactions, and imine addition reactions to forge new carbon–carbon bonds. These reactions enable the synthesis of a diverse array of valuable chemical scaffolds, including ethers, alcohols, 2,3-dihydrofurans, α-cyano-γ-butyrolactones, γ-butyrolactones, allylic compounds, gem-difluoroalkenes, β-amino ethers, and β-amino alcohols. Additionally, we have demonstrated the selective rearrangement of oxaziridines into nitrones and amides under photocatalytic conditions, with the formation of nitrones being solvent-dependent (acetonitrile, ethyl acetate, and acetone) and amides being formed through a weak-base-promoted rearrangement utilizing bases like CF3CO2− and DMF. Our ongoing research focuses on refining silicon-based alkyl radical precursors for the generation and application of various alkyl radicals and exploring further chemical transformations of oxaziridines. We are hopeful that our research will contribute to the expansion of eco-friendly organic synthesis.
References
1. (a) Narayanam JMR, Stephenson CRJ. 2011. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 40(1):102-113. https://doi.org/10.1039/B913880N (b) Tucker JW, Stephenson CRJ. 2012. Shining Light on Photoredox Catalysis: Theory and Synthetic Applications. J. Org. Chem. 77(4):1617-1622. https://doi.org/10.1021/jo202538x (c) Xuan J, Xiao WJ. 2012. Visible-Light Photoredox Catalysis. Angew. Chem., Int. Ed. 51(28):6828-6838. https://doi.org/10.1002/anie.201200223 (d) Prier CK, Rankic DA. MacMillan DWC. 2013. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113(7):5322-5363. https://doi.org/10.1021/cr300503r (e) Fukuzumi S, Ohkubo K. 2014. Organic synthetic transformations using organic dyes as photoredox catalysts. Org. Biomol. Chem. 12(32):6059-6071. https://doi.org/10.1039/c4ob00843j (f) Romero NA, Nicewicz DA. 2016. Organic Photoredox Catalysis. Chem. Rev. 116(17):10075-10166. https://doi.org/10.1021/acs.chemrev.6b00057 (g) Shaw MH, Twilton J, MacMillan DWC. 2016. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 81(16):6898-6926. https://doi.org/10.1021/acs.joc.6b01449 (h) Shang TY, Lu LH, Cao Z, Liu Y, He WM, Yu B. 2019. Recent advances of 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) in photocatalytic transformations. Chem. Commun. 55(38):5408-5419. https://doi.org/10.1039/c9cc01047e (i) Crespi S, Fagnoni M. 2020. Generation of Alkyl Radicals: From the Tyranny of Tin to the Photon Democracy. Chem. Rev. 120(17):9790-9833. https://doi.org/10.1021/acs.chemrev.0c00278 (j) Gontala A, Jang GS, Woo SK. 2021. Visible‐Light Photoredox‐Catalyzed α‐Allylation of α‐Bromocarbonyl Compounds Using Allyltrimethylsilane. Bull. Korean Chem. Soc. 42(3):506-509. https://doi.org/10.1002/bkcs.12219
2. Rehm D, Weller A. 1970. Kinetics of Fluorescence Quenching by Electron and H-Atom Transfer. Isr. J. Chem. 8(2):259-271. https://doi.org/10.1002/ijch.197000029
Aladdin:https://www.aladdinsci.com
