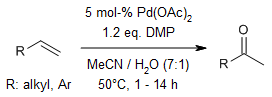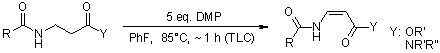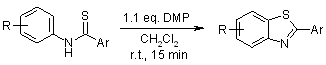
Product Manager:Nick Wilde
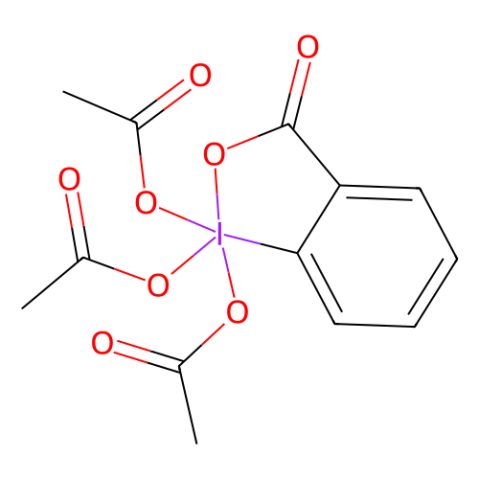
Dess-Martin periodinane (DMP) is a commercially available chemical that decomposes slowly. However, it is sensitive to heat and shock, and exhibits an exothermic reaction when heated above 130 °C. 2-Iodoxybenzoic acid (IBX), which is an impact-sensitive intermediate in the synthesis of DMP, is available in a DMSO solution and serves as an oxidizing agent as well.

Name Reactions
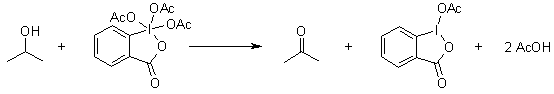
Dess-Martin Oxidation
Recent Literature
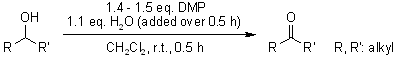
Partial hydrolysis or incomplete acetylation of DMP results in a more potent oxidant, which is why impure samples of DMP often yield better results than pure reagent. When a reliable and practical increase in reaction rate is needed, pure DMP can be decomposed with an equivalent amount of water just before or during its application.
S. D. Meyer, S. L. Schreiber, J. Org. Chem., 1994, 59, 7549-7552.
https://doi.org/10.1021/jo00103a067
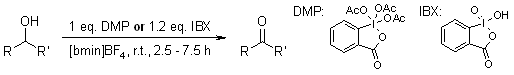
At room temperature, alcohols can be oxidized to their corresponding carbonyl compounds using 2-Iodoxybenzoic acid (IBX) or Dess-Martin periodinane (DMP) in the presence of [bmim]BF4 and [bmim]PF6 ionic liquids, resulting in high yields and selectivity. These reactions proceed faster in ionic liquids compared to traditional solvents. Additionally, the byproduct iodosobenzoic acid (IBA) and the ionic liquid can be easily recovered.
J. S. Yadav, B. V. S. Reddy, A. K. Basak, A. V. Narsaiah, Tetrahedron, 2004, 60, 2131-2135.
https://doi.org/10.1016/j.tet.2003.12.056
A combination of Pd(II) and Dess-Martin periodinane initiates a mimic of the Wacker process, converting terminal olefins into methyl ketones. This straightforward and scalable method exhibits Markovnikov selectivity, demonstrates good compatibility with functional groups, and is gentle yet highly productive.
D. A. Chaudhari, R. A. Fernandes, J. Org. Chem., 2016, 81, 2113-2121.
https://doi.org/10.1021/acs.joc.6b00137
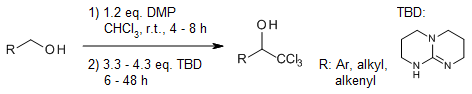
Primary alcohols can be converted into versatile trichloromethyl carbinols in a single-step process by treating them with Dess-Martin periodinane in CHCl3, followed by the addition of 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD).
M. K. Gupta, Z. Li, T. S. Snowden, J. Org. Chem., 2012, 77, 4854-4860.
https://doi.org/10.1021/jo300725v
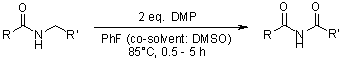
Among the reported instances of DMP, a hypervalent iodine reagent, exhibiting new reactivity are the processes of one-step oxidation of secondary amides to imides, N-acyl vinylogous carbamates, or ureas, as well as the direct oxidation of benzylic and related primary amines to their corresponding nitriles.
K. C. Nicolaou, C. J. N. Mathison, Angew. Chem. Int. Ed., 2005, 44, 5992-5997.
https://doi.org/10.1002/anie.200501853
K. C. Nicolaou, C. J. N. Mathison, Angew. Chem. Int. Ed., 2005, 44, 5992-5997.
https://doi.org/10.1002/anie.200501853
K. C. Nicolaou, C. J. N. Mathison, Angew. Chem. Int. Ed., 2005, 44, 5992-5997.
https://doi.org/10.1002/anie.200501853
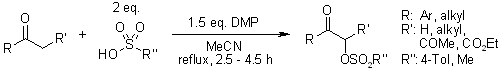
When combined with organosulfonic acids and heated to reflux temperature in acetonitrile, Dess-Martin periodinane reacts with ketones and dicarbonyl compounds to produce α-organosulfonyloxylated compounds in high yields.
U. S. Mahajan, K. G. Akamanchi, Synlett, 2008, 987-990.
https://doi.org/10.1055/s-2008-1072508
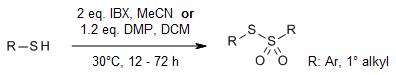
At room temperature, 2-Iodoxybenzoic acid (IBX) and Dess-Martin periodinane (DMP) can catalyze the transformation of thiols into thiosulfonates. Compared to IBX, DMP offers advantages in terms of faster reaction rates, higher conversions, and reduced equivalents needed. While IBX-catalyzed oxidation of benzyl thiols results in thiosulfonates, DMP leads to the formation of O-benzyl esters.
A. Chandra, N. Yadav, S. Payra, K. N. Parida, Org. Lett., 2023, 25, 6256-6261.
https://doi.org/10.1021/acs.orglett.3c02017
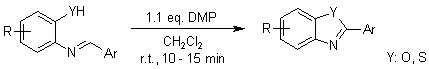
At ambient temperature, Dess-Martin periodinane (DMP) effectively catalyzes the intramolecular cyclization of phenolic azomethines, yielding substituted benzoxazoles and benzothiazoles. To obtain pure products, the reaction mixtures can be treated consecutively with Amberlyst A-26 thiosulfate resin and diisopropylaminomethyl resin (PS-DIEA), which remove excess reagent and byproducts.
D. S. Bose, M. Idrees, Synthesis, 2010, 398-402.
https://doi.org/10.1055/s-0029-1217136
A novel, gentle, and efficient approach for synthesizing 2-substituted benzothiazoles involves the intramolecular cyclization of thioformanilides using hypervalent iodine reagents in dichloromethane (CH2Cl2) at room temperature.
D. S. Bose, M. Idrees, J. Org. Chem., 2006, 71, 8261-8263.
https://doi.org/10.1021/jo0609374
Quoted from:
https://www.organic-chemistry.org/chemicals/oxidations/dess-martin-periodinane.shtm
Aladdin:https://www.aladdinsci.com