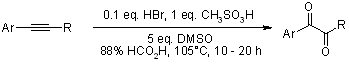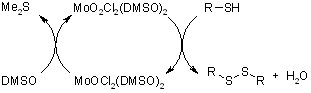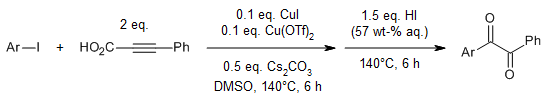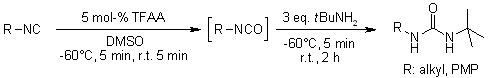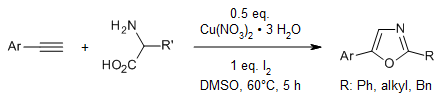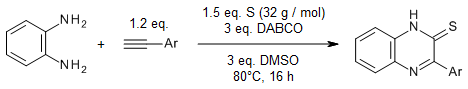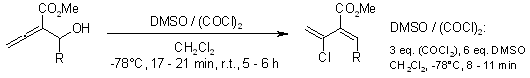Product Manager:Nick Wilde

Name Reactions
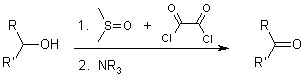
Swern Oxidation
Recent Literature
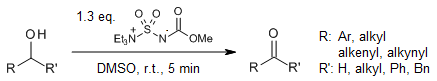
In the presence of dimethyl sulfoxide, the Burgess reagent efficiently and rapidly facilitates the oxidation of a broad range of primary and secondary alcohols to their corresponding aldehydes and ketones in excellent yields and under mild conditions. This oxidation can be combined with Wittig olefinations. A mechanism similar to those described for the Pfitzner-Moffatt and Swern oxidations is proposed.
P. R. Sultane, C. W. Bielawski, J. Org. Chem., 2017, 82, 1046-1052.
https://doi.org/10.1021/acs.joc.6b02629
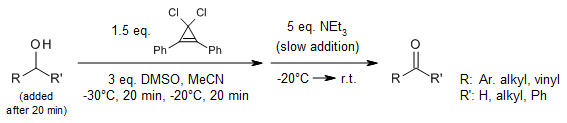
Swern oxidation using the volatile oxalyl chloride as an activator requires reaction temperatures below -60 °C. 3,3-Dichloro-1,2-diphenylcyclopropene (DDC) can be used as a new activator at -20°C. This convenient new protocol offers mild and fast reactions. Furthermore, the activator DDC is easy to handle, and diphenylcyclopropenone can be recovered quantitively.
T. Guo, Y. Gao, Z. Li, J. Liu, K. Guo, Synlett, 2019, 30, 329-332.
https://doi.org/10.1055/s-0037-1611183
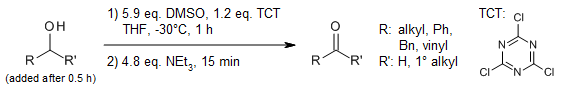
A mild and efficient alternative procedure for a quantitative conversion of alcohols into the corresponding carbonyl compounds uses dimethyl sulfoxide (DMSO), activated by Cyanuric chloride (TCT) instead of the toxic and moisture sensitive oxalyl chloride under Swern conditions.
L. De Luca, G. Giacomelli, A. Porcheddu, J. Org. Chem., 2001, 66, 7907-7909.
https://doi.org/10.1021/jo015935s
The use of DMSO in the presence of H2SO4 enables an efficient metal-free oxidation of benzylic alcohols to aromatic aldehydes in excellent yields. This oxidation proceeds in short reaction time without side products.
E. Sheikhi, M. Adib, M. A. Karajabad, S. J. A. Gohari, Synlett, 2018, 29, 974-978.
https://doi.org/10.1055/s-0037-1609149
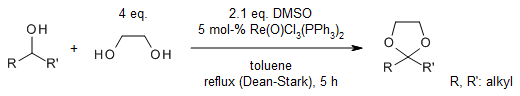
ReOCl3(PPh3)2 catalyzes a rapid oxidation of secondary alcohols by DMSO in the presence of ethylene glycol and refluxing toluene to provide the corresponding ketals in very good yields. Methyl sulfide and water as byproducts of the reaction are easily removed.
J. B. Arterburn, M. C. Perry, Org. Lett., 1999, 1, 769-771.
https://doi.org/10.1021/ol990755e
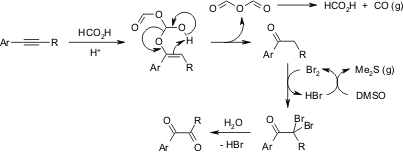
Oxidation of alkynes to α-dicarbonyl derivatives through a convenient one-pot procedure via a Brønsted acid-promoted "hydration" and a DMSO-based oxidation sequence has been achieved in high yields.
Z. Wan, C. D. Jones, D. Mitchell, J. Y. Pu, T. Y. Zhang, J. Org. Chem., 2006, 71, 826-828.
https://doi.org/10.1021/jo051793g
Selective and quantitative conversion of thiols to disulfides was effected by dimethyl sulfoxide under mild conditions catalyzed by dichlorodioxomolybdenum(VI).
R. Sanz, R. Aguado, M. R. Pedrosa, F. Arnáiz, Synthesis, 2002, 856-858.
https://doi.org/10.1055/s-2002-28520
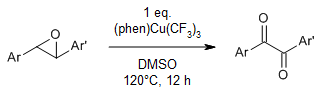
A sequential dehydrogenation and transfer oxygenation of 1,2-diarylepoxides by high-valent phenCu(III)(CF3)3 and DMSO provides 1,2-diketones. In situ generated CF3 radicals abstract the hydrogen atom of the epoxide ring. The resulting ether α-carbon radical undergoes ring-opening rearrangement to give a ketone α-carbon radical intermediate, which is oxygenated by DMSO.
D.-D. Chen, S.-L. Zhang, J. Org. Chem., 2023, 88, 16735-16741.
https://doi.org/10.1021/acs.joc.3c01160
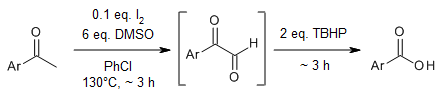
A metal-free and one-pot two-step synthesis of aryl carboxylic acids from aryl alkyl ketones has been performed with iodine as the catalyst, DMSO and TBHP as the oxidants. Various aryl alkyl ketones could be converted into their corresponding aryl carboxylic acids in very good yields.
L. Xu, S. Wang, B. Chen, M. Li, X. Hu, B. Hu, L. Jin, N. Sun, Z. Shen, Synlett, 2018, 29, 1505-1509.
https://doi.org/10.1055/s-0037-1609751
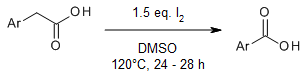
An I2-promoted direct conversion of arylacetic acids into aryl carboxylic acids under metal-free conditions involves decarboxylation followed by an oxidation reaction enabled just by using DMSO as the solvent as well as an oxidant. Notably, aryl carboxylic acids are isolated by simple filtration technique and obtained in good to excellent yields, which makes this protocol applicable for large-scale synthesis.
H. P. Kalmode, K. S. Vadagaonkar, S. L. Shinde, A. C. Chaskar, J. Org. Chem., 2017, 82, 3781-3786.
https://doi.org/10.1021/acs.joc.7b00242
Benzil derivatives such as diaryl 1,2-diketones are synthesized via a direct copper-catalyzed decarboxylative coupling reaction of aryl propiolic acids with aryl iodides followed by an oxidation. The reaction shows good functional group tolerance toward ester, aldehyde, cyano, and nitro groups. In addition, symmetrical diaryl 1,2-diketones are obtained from aryl iodides and propiolic acid in the presence of palladium and copper catalysts.
H. Min, T. Palani, K. Park, J. Hwang, S. Lee, J. Org. Chem., 2014, 79, 6279-6285.
https://doi.org/10.1021/jo501089k
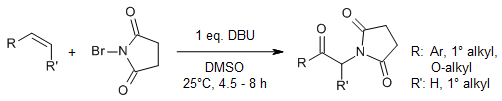
An oxo-amination process with readily available N-bromosuccinimide (NBS) and secondary amines as N-sources and dimethyl sulfoxide (DMSO) as the oxidant provides amino alcohols in a single step. For the first time, the formation of reactive Me2S+-O-Br species generated by the interaction of NBS with DMSO has been proven.
P. K. Prasad, R. N. Reddi, A. Sudalai, Org. Lett., 2016, 18, 500-503.
https://doi.org/10.1021/acs.orglett.5b03540
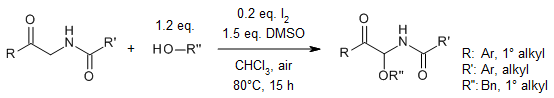
An I2-catalyzed oxidative cross-coupling of α-amino ketones with a wide range of alcohols provides α-carbonyl N,O-acetals with high functional group tolerance. Using a combination of air and dimethyl sulfoxide as oxidants, the protocol allows an efficient late-stage modification of biorelevant structures. Moreover, the use of other nucleophiles enables additional functionalization of α-amino ketones.
Y. Wang, M. Yang, C. Lao, H. Wang, Z. Jiang, J. Org. Chem., 2023, 88, 14470-14486.
https://doi.org/10.1021/acs.joc.3c01469
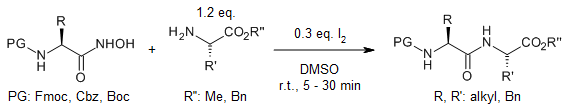
Formation of unstable but reactive acyl nitroso intermediates from Nα-protected hydroxamic acids in the presence of iodine and DMSO enables an efficient and straightforward coupling with an amino component to yield dipeptide esters.
M. Krishnamurthy, T. M. Vishwanatha, N. R. Panguluri, V. Panduranga, V. V. Sureshbabu, Synlett, 2015, 26, 2565-2569.
https://doi.org/10.1055/s-0035-1560266
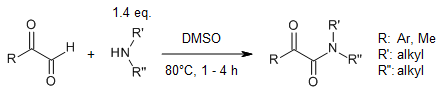
A dimethyl sulfoxide (DMSO)-promoted oxidative amidation reaction between 2-oxoaldehydes and amines under metal-free conditions enables an efficient synthesis of α-ketoamides. Mechanistic studies supported an iminium ion intermediate that reacts with DMSO to provide the C1-oxygen atom of the product.
N. Mupparapu, S. Khan, S. Battula, M. Kushwaha, A. P. Gupta, Q. N. Ahmed, R. A. Vishwakarma, Org. Lett., 2014, 16, 1152-1155.
https://doi.org/10.1021/ol5000204
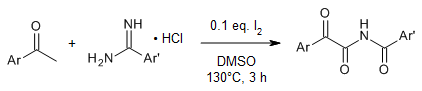
An iodine-catalyzed oxidative C-H/N-H cross-coupling enables an efficient construction of α-ketoimides in good to excellent yields from methyl ketones and benzamidines hydrochloride under metal-free and peroxide-free conditions.
X. Wu, Q. Gao, S. Liu, A. Wu, Org. Lett., 2014, 16, 2888-2891.
https://doi.org/10.1021/ol501029w
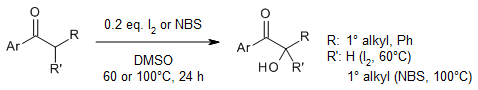
In an efficient α-hydroxylation of carbonyls compounds, readily available I2 or N-bromosuccinimide (NBS) was used as catalyst and DMSO as terminal oxidant. The reaction is mild, less toxic, easy to perform and allows the conversion of a diverse range of tertiary as well as secondary Csp3-H bonds.
Y.-F. Liang, K. Wu, S. Song, X. Li, X. Huang, N. Jiao, Org. Lett., 2015, 17, 876-879.
https://doi.org/10.1021/ol5037387
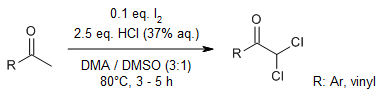
Methyl ketones can undergo dichlorination to afford α,α-dichloroketones in good yields with precise control of the chemoselectivity. Enabled by the I2-dimethyl sulfoxide catalytic system, in which hydrochloric acid only acts as a nucleophilic Cl- donor, this straightforward dichlorination reaction is safe and operator-friendly and has high atomic economy and good functional-group tolerance.
J.-C. Xiang, J.-W. Wang, P. Yuan, J.-T. Ma, A.-X. Wu, Z.-X. Liao, J. Org. Chem., 2022, 87, 15101-15113.
https://doi.org/10.1021/acs.joc.2c01591
A smooth and efficient oxidation of isonitriles to isocyanates by DMSO as the oxidant is catalyzed by trifluoroacetic anhydride. The process is complete in a few minutes, forming dimethyl sulfide as the only byproduct. The newly formed isocyanates may be used directly or isolated in high purity by solvent evaporation.
H. V. Le, B. Ganem, Org. Lett., 2011, 13, 2584-2585.
https://doi.org/10.1021/ol200695y
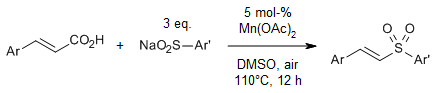
Reactions of cinnamic acids with aromatic sulfinic acid sodium salts in the presence of a catalytic amount of manganese(II) acetate tetrahydrate in dimethyl sulfoxide provide vinyl sulfones in very good yields. The use of DMSO as solvent and the presence of air are critical in achieving good yields.
N. Xue, R. Guo, X. Tu, W. Luo, W. Deng, J. Xiang, Synlett, 2016, 27, 2695-2698.
https://doi.org/10.1055/s-0035-1562476
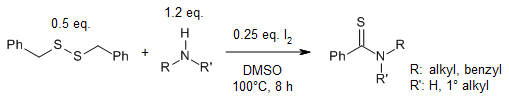
A metal- and additive-free reaction of 1,2-dibenzyldisulfanes with amines using iodine as oxidant and DMSO as solvent at 100°C provides various thioamides in high yields.
S. Chen, Y. Li, J. Chen, X. Xu, L. Su, Z. Tang, C.-T. Au, R. Qiu, Synlett, 2016, 27, 2339-2344.
https://doi.org/10.1055/s-0035-1562509
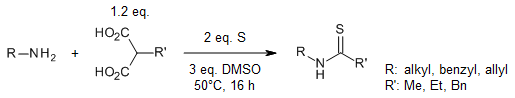
Malonic acid and derivatives can be used as C1 synthons via double decarboxylation promoted by sulfur and dimethyl sulfoxide. In the presence of amines as nucleophiles, a wide range of thioureas and thioamides as well as N-heterocycles were obtained in good yields under mild heating conditions.
T. H. Do, S. Phaenok, D. Soorukram, T. Modjinou, D. Grande, T. T. T. Nguyen, T. B. Nguyen, Org. Lett., 2023, 25, 6322-6327.
https://doi.org/10.1021/acs.orglett.3c02247
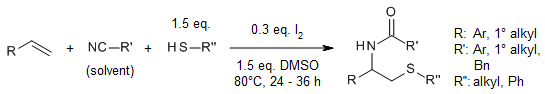
A direct difunctionalization protocol of alkenes with nitriles and thiols under metal-free synthesis conditions provides various β-acetamido sulfides with very good yields simply by using inexpensive molecular iodine as a catalyst, DMSO as a mild oxidant, and readily available thiols as thiolating reagents.
H. Cui, X. Liu, W. Wei, D. Yang, C. He, T. Zhang, H. Wang, J. Org. Chem., 2016, 81, 2252-2260.
https://doi.org/10.1021/acs.joc.5b02579
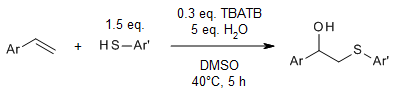
A convenient and metal-free three-component oxychalcogenation reaction of alkenes, diselenides/thiophenols, and H2O/alcohols provides β-hydroxyl or β-alkoxy organochalcogenides in very good yields. Tetrabutylammonium tribromide (TBATB) and dimethyl sulfoxide (DMSO) are utilized as the catalyst and the terminal oxidant, respectively.
J. Huang, X. Li, L. Xu, Y. Wei, J. Org. Chem., 2023, 88, 3054-3067.
https://doi.org/10.1021/acs.joc.2c02856
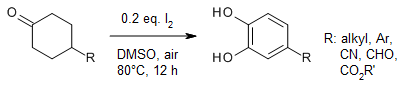
In a I2-catalyzed direct conversion of cyclohexanones to substituted catechols under mild and simple conditions via multiple oxygenation and dehydrogenative aromatization processes, DMSO acts as the solvent, oxidant, and oxygen source. This metal-free and simple system provides highly valuable substituted catechols for drug discovery.
Y.-F. Liang, X. Li, X. Wang, M. Zou, C. Tang, Y. Liang, S. Song, N. Jiao, J. Am. Chem. Soc., 2016, 138, 12271-12277.
https://doi.org/10.1021/jacs.6b07269
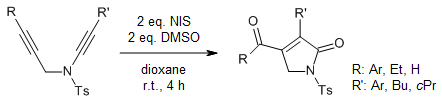
Dimethyl sulfoxide (DMSO) and N-iodosuccinimide mediate a metal-free regioselective 5-exo-dig oxidative cyclization of propargyl-substituted ynamides via in situ generated enol equivalents. The reaction allows easy access to functionalized pyrrolidone skeletons. The role of DMSO as oxidant in the transformation is clarified, and a tentative reaction pathway is proposed.
B. Prabagar, S. Nayak, R. Prasad, A. K. Sahoo, Org. Lett., 2016, 18, 3066-3069.
https://doi.org/10.1021/acs.orglett.6b01149
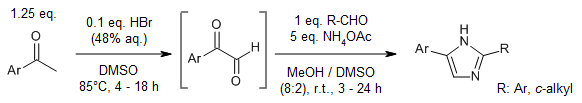
A ketone oxidation, employing catalytic HBr and DMSO, followed by imidazole condensation with aldehydes provides 2,4(5)-disubstituted imidazoles in good yields. A three-step synthesis of 20 kinase inhibitors was achieved by employing this oxidation-condensation protocol, followed by bromination and Suzuki coupling.
I. de Toledo, T. A. Grigolo, J. M. Bennett, J. M. Elkins, R. A. Pilli, J. Org. Chem., 2019, 84, 14187-14201.
https://doi.org/10.1021/acs.joc.9b01844
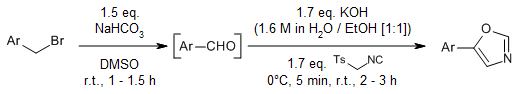
A One-Pot Tandem Approach for the Synthesis of 5-(Het)aryloxazoles from Substituted (Het)aryl Methyl Alcohols and Benzyl Bromides
K. S. V. Kumar, T. R. Swaroop, N. Rajeev, A. C. Vinayaka, G. S. Lingaraju, K. S. Rangappa, M. P. Sadashiva, Synlett, 2016, 27, 1363-1366.
https://doi.org/10.1055/s-0035-1561391
2,5-disubstituted oxazoles can be synthesized from easily available arylacetylenes and α-amino acids in the presence of Cu(NO3)2•3H2O and iodine. This reaction involves an I2/Cu(NO3)2•3H2O-assisted generation of α-iodo acetophenones, a Kornblum oxidation to phenylglyoxals, a condensation to imines, and a decarboxylation/annulation/oxidation reaction sequence.
J. Wang, Y. Cheng, J. Xiang, A. Wu, Synlett, 2019, 30, 743-747.
https://doi.org/10.1055/s-0037-1612087
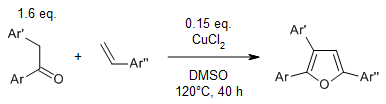
A copper-catalyzed oxidative cyclization of aryl ketones with styrenes to furans, in which DMSO serves not only as a solvent but also as an oxidant, provides multiaryl-substituted furans from cheap and readily available starting materials.
Y. Wu, Z. Huang, Y. Luo, D. Liu, Y. Deng, H. Yi, J.-Fu. Lee, C.-W. Pao, J.-L. Chen, A. Lei, Org. Lett., 2017, 19, 2330-2333.
https://doi.org/10.1021/acs.orglett.7b00865
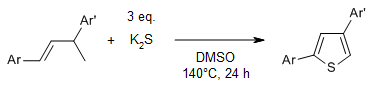
The reaction of substituted buta-1-enes with potassium sulfide enables an atom economical, and transition-metal-free synthesis of thiophenes via cleavage of multiple C-H bonds. Moreover, the strategy can also be used for the synthesis of thiophenes from 1,4-diaryl-1,3-dienes.
L. Chen, H. Min, W. Zeng, X. Zhu, Y. Liang, G. Deng, Y. Yang, Org. Lett., 2018, 20, 7392-7395.
https://doi.org/10.1021/acs.orglett.8b03078
The reaction of substituted buta-1-enes with potassium sulfide enables an atom economical, and transition-metal-free synthesis of thiophenes via cleavage of multiple C-H bonds. Moreover, the strategy can also be used for the synthesis of thiophenes from 1,4-diaryl-1,3-dienes.
L. Chen, H. Min, W. Zeng, X. Zhu, Y. Liang, G. Deng, Y. Yang, Org. Lett., 2018, 20, 7392-7395.
https://doi.org/10.1021/acs.orglett.8b03078
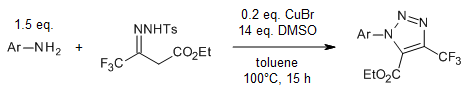
Copper catalyzes a rapid assembly of 5-carboxyl-4-perfluoroalkyl-triazoles from N-tosylhydrazides and perfluoroalkyl acetoacetates. The approach exhibits high functional group tolerance and can be executed on a multigram scale.
R. Panish, T. Thieu, J. Balsells, Org. Lett., 2021, 23, 5937-5941.
https://doi.org/10.1021/acs.orglett.1c02037
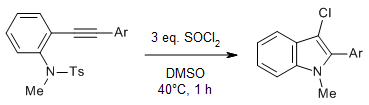
An intramolecular cyclization/chlorination of N,N-disubstituted 2-alkynylanilines provides a series of 3-chloroindoles with good yields using the DMSO/SOCl2 system. The incorporation of the chlorine atom is realized via a desulfonylative chlorocyclization process.
X. Li, Y. Cheng, Y. Li, F. Sun, X. Zhan, Z. Yang, J. Yang, Y. Du, J. Org. Chem., 2024, 89, 2039-2049.
https://doi.org/10.1021/acs.joc.3c02471
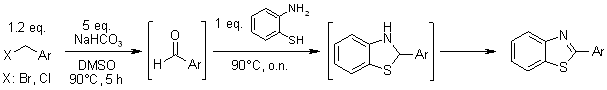
A one-pot tandem reaction of benzyl halides and o-aminobenzenethiol gives benzothiazoles in high chemical yields under mild conditions in DMSO in the absence of an additional oxidant. Both benzyl chlorides and bromides bearing a range of substituents proved to be suitable substrates.
C. Zhu, T. Akiyama, Synlett, 2010, 2345-2351.
https://doi.org/10.1055/s-0030-1258046
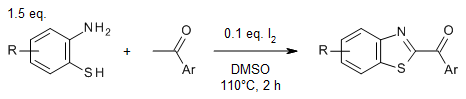
Iodine catalyzes simple and practical syntheses of 2-aroylbenzothiazoles and 2-arylbenzothiazoles from 2-aminobenzenethiols and acetophenones under metal-free conditions. Reactions in DMSO as oxidant and reaction medium provide 2-aroylbenzothiazoles, whereas the use of nitrobenzene as oxidant in dioxane as solvent enables the synthesis of 2-arylbenzothiazoles.
R. Ma, Y. Ding, R. Chen, Z. Wang, L. Wang, Y. Ma, J. Org. Chem., 2021, 86, 310-321.
https://doi.org/10.1021/acs.joc.0c02095
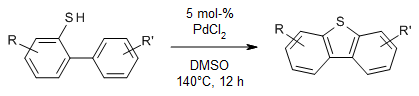
A simple catalytic system, in which PdCl2 is the sole metal catalyst and DMSO functions as an oxidant and solvent, enables a synthesis of dibenzothiophenes in high yields from 2-biphenylthiols. This transformation offers broad substrate scope and operational simplicity.
T. Zhang, G. Deng, H. Li, B. Liu, Q. Tan, B. Xu, Org. Lett., 2018, 20, 5439-5443.
https://doi.org/10.1021/acs.orglett.8b02347
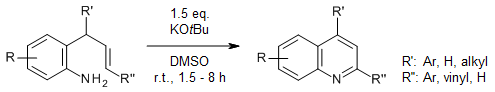
A regioselective 6-endo-trig intramolecular oxidative cyclization enabled an efficient synthesis of 2-aryl 4-substituted quinolines from stable and readily available o-cinnamylanilines with KOtBu as a mediator and DMSO as an oxidant at rt. The reaction showed a broad substrate scope with very good yields.
M. Rehan, G. Hazra, P. Ghorai, Org. Lett., 2015, 17, 1668-1671.
https://doi.org/10.1021/acs.orglett.5b00419
A visible-light-mediated oxidative cyclization of 2-aminobenzyl alcohols with secondary alcohols provides quinolines in good yields at room temperature. This photocatalytic method employes anthraquinone as an organic small-molecule catalyst and DMSO as an oxidant.
J.-x. Xu, N.-l. Pan, J.-x. Chen, J.-w. Zhao, J. Org. Chem., 2021, 86, 10747-10754.
https://doi.org/10.1021/acs.joc.1c01386
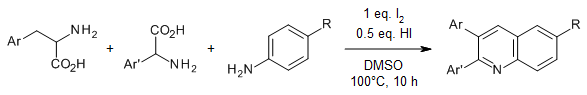
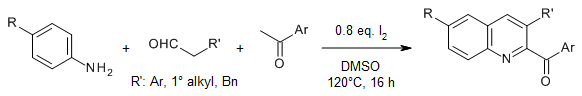
A practicable quinoline synthesis from aniline and two amino acids provides a wide range of quinolines with high efficiency and diversity including pharmaceutical derivatives, photochemical active compounds, and challenging scaffolds. Mechanistic studies revealed that I2 promotes decarboxylation, oxidative deamination, and selective formation of new C-N and C-C bonds.
J.-C. Xiang, Z.-X. Wang, Y. Cheng, S.-Q. Xia, M. Wang, B.-C. Tang, Y.-D. Wu, A.-X. Wu, J. Org. Chem., 2017, 82, 9210-9216.
https://doi.org/10.1021/acs.joc.7b01501
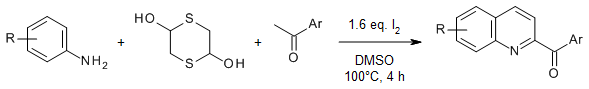
A highly efficient I2-promoted formal [4+2] cycloaddition enables the synthesis of 2-acylquinolines from methyl ketones and arylamines using 1,4-dithane-2,5-diol as an ethylene surrogate. This reaction occurred via an iodination/Kornblum oxidation/Povarov/aromatization sequence with an important role of the arylamine substrate in promoting the reaction.
X. Wu, X. Geng, P. Zhao, J. Zhang, X. Gong, Y.-d. Wu, A.-x. Wu, Org. Lett., 2017, 19, 1550-1553.
https://doi.org/10.1021/acs.orglett.7b00361
A synergistic I2/amine promoted formal [4+2] cycloaddition of methyl ketones, arylamines, and aryl(alkyl)acetaldehydes provides various 2-acyl-3-aryl(alkyl)quinolines via an iodination/Kornblum oxidation/Povarov/aromatization sequence. Notably, the arylamine reactants also acted as indispensable catalysts to promote enamine formation.
X. Geng, X. Wu, P. Zhao, J. Zhang, Y.-D. Wu, A.-X. Wu, Org. Lett., 2017, 19, 4179-4182.
https://doi.org/10.1021/acs.orglett.7b01686
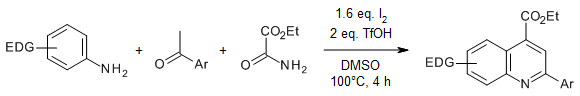
An efficient [2+1+3] cyclization reaction of aryl methyl ketones, arylamines, and 1,3-dicarbonyl compounds provides 2-aryl-4-quinolinecarboxylates in good yields under mild conditions. This metal-free process achieved a C-C bond cleavage of 1,3-dicarbonyl compounds for use as a C1 synthon.
Y. Zhou, P. Zhao, L.-S. Wang, X.-X. Yu, C. Huang, Y. D. Wu, A.-X. Wu, Org. Lett., 2021, 23, 6461-6465.
https://doi.org/10.1021/acs.orglett.1c02267
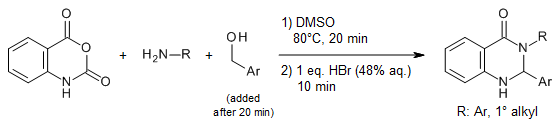
The reaction between isatoic anhydride, primary amines, and benzylic alcohols in the presence of HBr in DMSO at 80°C affords 2,3-dihydroquinazolin-4(1H)-ones in excellent yields via a metal-free oxidative C(sp3)-N coupling process.
N. R. E. Sheikhi, P. R. Ranjbar, Synlett, 2018, 29, 912-917.
https://doi.org/10.1055/s-0036-1591544
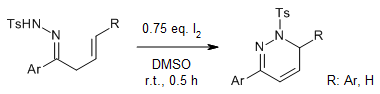
Iodine catalyzes a highly efficient and chemoselective oxidative annulation of β,γ-unsaturated hydrazones to produce 1,6-dihydropyridazines under mild conditions. When active β,γ-unsaturated hydrazone compounds containing electron-donating groups, such as furyl, thienyl, and cycloalkyl, were used, pyrroles were obtained.
Q. Liu, J. Jiang, X. Ye, J. Sun, Y. Wu, Y. Shao, C. Deng, F. Zhang, J. Org. Chem., 2023, 88, 10632-10646.
https://doi.org/10.1021/acs.joc.3c00669
DABCO serves as a sulfur-activating catalyst to achieve the sulfurative 1,2-diamination of phenylacetylenes with elemental sulfur and o-phenylenediamines in the presence of DMSO as terminal oxidant. This cascade three-component reaction is triggered by the addition of active sulfur species to the triple bond of phenylacetylenes.
T. M. C. Tran, N. D. Lai, T. T. T. Bui, D. H. Mac, T. T. T. Nguyen, P. Retailleau, T. B. Nguyen, Org. Lett., 2023, 25, 7186-7191.
https://doi.org/10.1021/acs.joc.3c00669
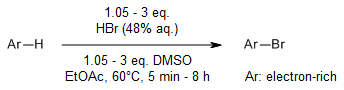
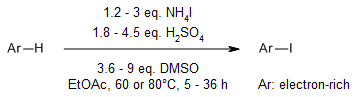
DMSO as a mild and inexpensive oxidant enables an efficient and practical bromination and iodination of arenes with HX (X = Br, I) reagents. This oxidative system is amenable to late-stage bromination of natural products and kilogram-scale conversions.
S. Song, X. Sun, X. Li, Y. Yuan, N. Jiao, Org. Lett., 2015, 17, 2886-2889.
https://doi.org/10.1021/acs.orglett.5b00932
S. Song, X. Sun, X. Li, Y. Yuan, N. Jiao, Org. Lett., 2015, 17, 2886-2889.
https://doi.org/10.1021/acs.orglett.5b00932
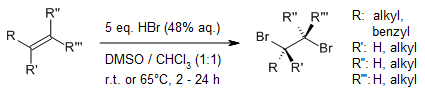
In an oxidative bromination of alkenes to 1,2-dibromo alkanes with HBr, dimethyl sulfoxide serves as the oxidant as well as cosolvent. Whereas simple olefins are brominated in very good yields, three of six styrene derivatives yielded bromohydrins under the reaction conditions.
M. Karki, J. Magolan, J. Org. Chem., 2015, 80, 3701-3707.
https://doi.org/10.1021/acs.joc.5b00211
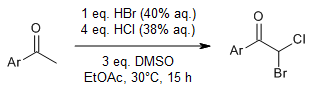
The combination of dimethyl sulfoxide, HCl, and HBr enables a mild, efficient, and practical geminal heterodihalogenation of methyl ketones. This convenient method might be useful for the assembly of bromochloromethyl groups in drug discovery.
J.-f. Zhou, D.-m. Tang, M. Bian, Synlett, 2020, 31, 1430-1434.
https://doi.org/10.1055/s-0040-1707169
Highly regio- and stereoselective reactions of readily available 2-(methoxycarbonyl)-2,3-allenols with oxalyl chloride in the presence of Et3N or DMSO afforded methyl 2-(ethynyl)alk-2(E)-enoates and 2-(1′-chlorovinyl)alk-2(Z)-enoates, respectively, in good yields.
Y. Deng, X. Kin, C. Fu, S. Ma, Org. Lett., 2009, 11, 2169-2172.
https://doi.org/10.1021/ol9004273
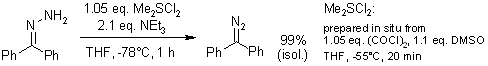
"Activated" dimethyl sulfoxide efficiently dehydrogenates hydrazones to the respective diazo compounds at -78°C. Under optimized conditions, simple vacuum filtration provides solutions of pure diazo compounds from which stable diazo species can be isolated in high yield, or that can be directly used in subsequent reactions.
M. I. Javed, M. Brewer, Org. Lett., 2007, 9, 1789-1792.
https://doi.org/10.1021/ol070515w
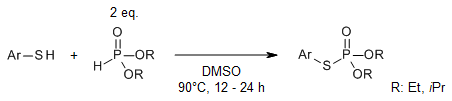
A convenient reaction of thiols with H-dialkyl phosphites in the presence of DMSO as both solvent and oxidant provides various phosphorothioates under transition-metal-free conditions. The reaction proceeds with formation of a disulfide intermediate followed by nucleophilic substitution with the dialkyl phosphite.
B. Kaboudin, P. Daliri, H. Esfandiari, F. Kazemi, Synlett, 2023, 34, 249-252.
https://doi.org/10.1055/a-1983-1640
Quoted from: https://www.organic-chemistry.org/chemicals/oxidations/dimethylsulfoxide.shtm