Product Manager:Nick Wilde

INTRODUCTION
The amide bond is one of the most commonly used chemical bonds in organic synthesis. The methods used to form this important functional group are often superchemically stoichiometric, leading to significant waste in the process. These factors led the American Chemical Society Green Chemistry Institute Pharmaceutical Roundtable (ACS-GCIPR) to highlight the catalytic synthesis of amide bonds as a significant initiative in green chemistry.1
Aladdin Scientific offers many related products for catalytic amidation technology.2
REPRESENTATIVE TRANSFORMATIONS
In 2007, Milstein and his team described the dehydrogenative coupling of amines and alcohols to form amides, utilizing a ruthenium catalyst.3
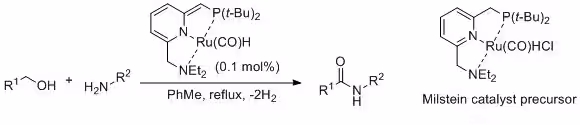
Later, Madsen and his collaborators introduced an alternative catalyst system for this dehydrogenative coupling, significantly expanding its scope.4
![]()
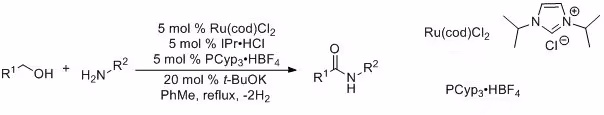
In more recent developments, Hall and colleagues showcased boronic acid-catalyzed amidation reactions employing carboxylic acids as substrates.3
References
1. Constable DJC, Dunn PJ, Hayler JD, Humphrey GR, Leazer, Jr. JL, Linderman RJ, Lorenz K, Manley J, Pearlman BA, Wells A, et al. Key green chemistry research areas a perspective from pharmaceutical manufacturers. Green Chem.. 9(5):411-420. https://doi.org/10.1039/b703488c
2. Pattabiraman VR, Bode JW. 2011. Rethinking amide bond synthesis. Nature. 480(7378):471-479. https://doi.org/10.1038/nature10702
3. Gunanathan C, Ben-David Y, Milstein D. 2007. Direct Synthesis of Amides from Alcohols and Amines with Liberation of H2. Science. 317(5839):790-792. https://doi.org/10.1126/science.1145295
4. Nordstrøm LU, Vogt H, Madsen R. 2008. Amide Synthesis from Alcohols and Amines by the Extrusion of Dihydrogen. J. Am. Chem. Soc.. 130(52):17672-17673. https://doi.org/10.1021/ja808129p
5. Al-Zoubi R, Marion O, Hall D. 2008. Direct and Waste-Free Amidations and Cycloadditions by Organocatalytic Activation of Carboxylic Acids at Room Temperature. Angew. Chem. Int. Ed.. 47(15):2876-2879. https://doi.org/10.1002/anie.200705468
Aladdin:https://www.aladdinsci.com

