Sandra Forbes
Product Manager
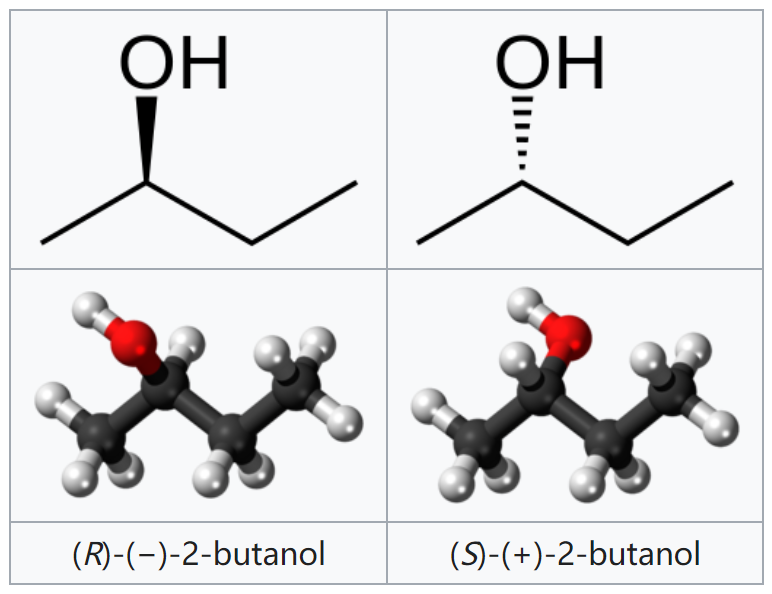
Butan-2-ol, or sec-butanol, is an organic compound with formula CH3CH(OH)CH2CH3. Its structural isomers are 1-butanol, isobutanol, and tert-butanol. 2-Butanol is chiral and thus can be obtained as either of two stereoisomers designated as (R)-(−)-butan-2-ol and (S)-(+)-butan-2-ol. It is normally encountered as a 1:1 mixture of the two stereoisomers — a racemic mixture.
This secondary alcohol is a flammable, colorless liquid that is soluble in three parts water and completely miscible with organic solvents. It is produced on a large scale, primarily as a precursor to the industrial solvent methyl ethyl ketone.
Manufacture and Applications
Butan-2-ol is manufactured industrially by the hydration of 1-butene or 2-butene:
![]()
Sulfuric acid is used as a catalyst for this conversion.
In the laboratory it can be prepared via Grignard reaction by reacting ethylmagnesium bromide with acetaldehyde in dried diethyl ether or tetrahydrofuran.
Although some butan-2-ol is used as a solvent, it is mainly converted to butanone (methyl ethyl ketone, MEK), an important industrial solvent and found in many domestic cleaning agents and paint removers. Though most paint removers have ceased using MEK in their products due to health concerns and new laws. Volatile esters of butan-2-ol have pleasant aromas and are used in small amounts as perfumes or in artificial flavors.
Recent Literature
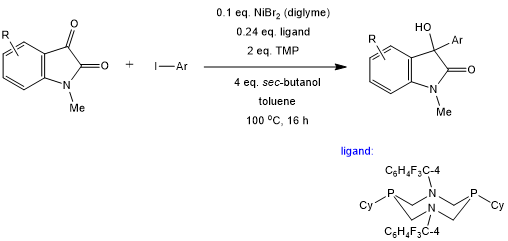
An intermolecular Ni-catalyzed reductive coupling of aryl iodides and isatins provides 3-hydroxyoxindoles in the presence of sec-butanol as a mild stoichiometric reductant. This formal 1,2-addition reaction is facilitated by a 1,5-diaza-3,7-diphosphacyclooctane (P2N2) ligand.
A. Nasim, G. T. Thomas, J. S. Ovens, S. G. Newman, Org. Lett., 2022, 24, 7232-7236.
DOI: 10.1021/acs.orglett.2c03042
Quoted from: https://www.organic-chemistry.org/chemicals/reductions/sec-butanol-2-butanol.shtm
Aladdinsci: https://www.aladdinsci.com/
