Flow cytometry is a very practical technology, which is characterized by convenience, accuracy of results and successful sorting of target cells, but mentioning how to choose flow antibodies, it is estimated that many partners will be difficult, indeed, it is not so easy to get a beautiful flow results graph, the most important thing to consider before the experiment is the selection of antibodies and fluorescein collocation.
What should I pay attention to when choosing flow-through antibodies?
Basic requirements of antibodies
a. Know clearly whether the target to be measured is an intracellular or extracellular protein;
b. Select antibody reactivity strictly according to the source of the sample;
c. It is necessary to choose antibodies that are clearly labeled for use in flow experiments and cannot be replaced by common antibodies.
Flow cytometry requirements
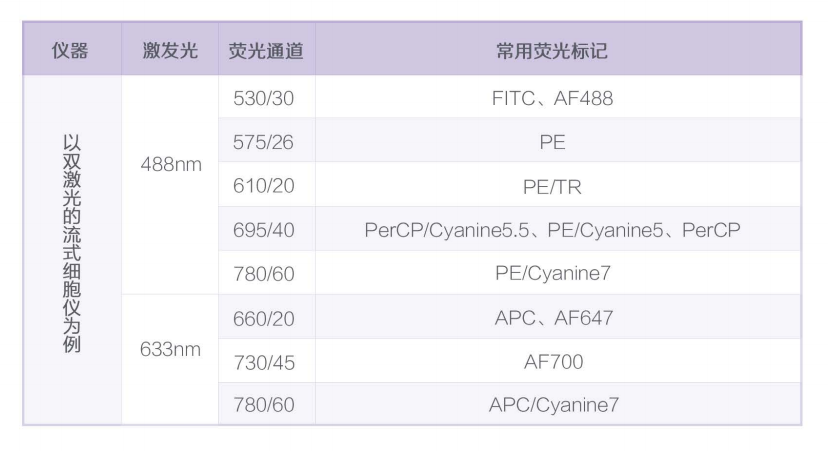
a. Know how many lasers are available for the flow cytometer used, common lasers are 405nm, 488nm and 633nm, etc. Currently most flow cytometers are equipped with 2 lasers, 488nm and 633nm;
b. Know how many detection channels are available under each laser, and the number of channels determines how many indicators will be measured at one time;
Selection of fluorescent labeling
a. If you have direct labeling antibody, try to choose direct labeling, indirect labeling secondary antibody increases the experimental steps, multiple washing will cause cell loss, staining accuracy is also low;
b. For weakly expressed targets, try to choose strong fluorescein labeled antibody, PE>APC>FITC>PerCP.
Multi-color matching scheme
a. Only one marker can be used for each channel, e.g. FITC and AF488, both markers have similar excitation and emission wavelengths, so you can't use both at the same time;
b. Minimize spectral overlap, e.g. PE-cy5 and APC, both are used together when the tuning compensation value is too large;
c. Weak targets with strong fluorescein, e.g. CD4 expression in Treg cells is higher than FoxP3, so FoxP3 should use strong fluorescein labeled antibody;
d. For color matching of five or more colors, it is recommended to consult the literature or the antibody manufacturer.
How to adjust compensation?
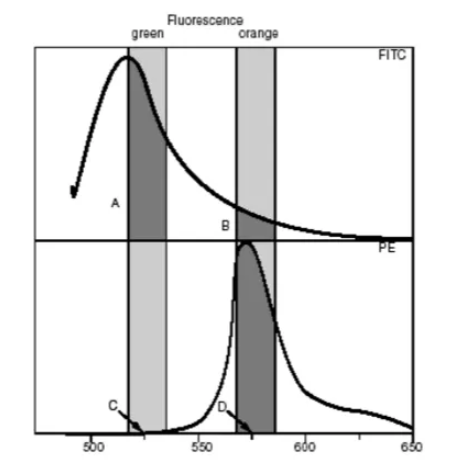
Multi-color flow experiments, due to the existence of spectral overlap of individual fluorescein, so compensation adjustment is required. The following figure shows the spectral diagram of two fluoresceins, FITC and PE, and there is spectral overlap of the two dyes near 550nm.
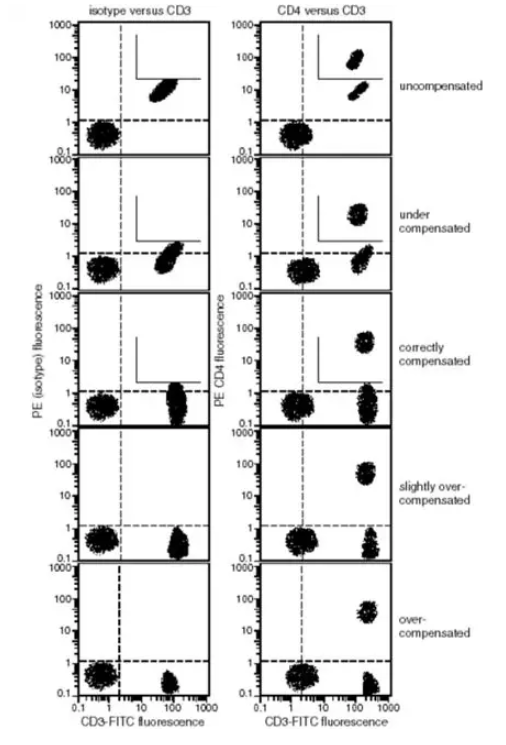
The following figure shows the regulation of staining compensation for CD3-FITC, CD4-PE
Isotype control?
The purpose of isotype control is to minimize background interference and to better demonstrate the binding strength of the antigen to the antibody, especially for some intracellular proteins, low-expressed or continuously expressed proteins, the setting of isotype control is especially necessary. For isotype control, the antibody should be of the same genus, subtype and fluorescent label as the stained monoclonal antibody.
For more information, visit our website: www.aladdinsci.com.
