Determine the necessary mass, volume, or concentration for preparing a solution.
This is a demo store. No orders will be fulfilled.
| SKU | Size | Availability | Price | Qty |
|---|---|---|---|---|
| rp170191-10μg | 10μg | Available within 8-12 weeks(?) Production requires sourcing of materials. We appreciate your patience and understanding. | $599.90 | |
| rp170191-20μg | 20μg | 3 | $999.90 |
EGF-Biotin, Active, Pichia pastoris, No tag, 971-1021 aa
| Product Name | Recombinant Human EGF Protein (Biotin) |
|---|---|
| Synonyms | beta-urogastrone | EGF | epidermal growth factor (beta-urogastrone) | epidermal growth factor | hEGF | HOMG4 | pro-epidermal growth factor | URG | Urogastrone | EGF |
| Grade | ActiBioPure™, Azide Free, Bioactive |
| Product Description | Introduction: |
| Specifications & Purity | ActiBioPure™, Azide Free, Bioactive |
| Bioactivity | Binding EGFR |
| Expression System | Pichia pastoris |
| Species | Human |
| Amino Acids | 971 - 1021 aa |
| Sequence | NSDSECPLSHDGYCLHDGVCMYIEALDKYACNCVVGYIGERCQYRDLKWWE |
| Protein Tag | No tag |
| Conjugation | Biotin |
| Accession # | P01133 |
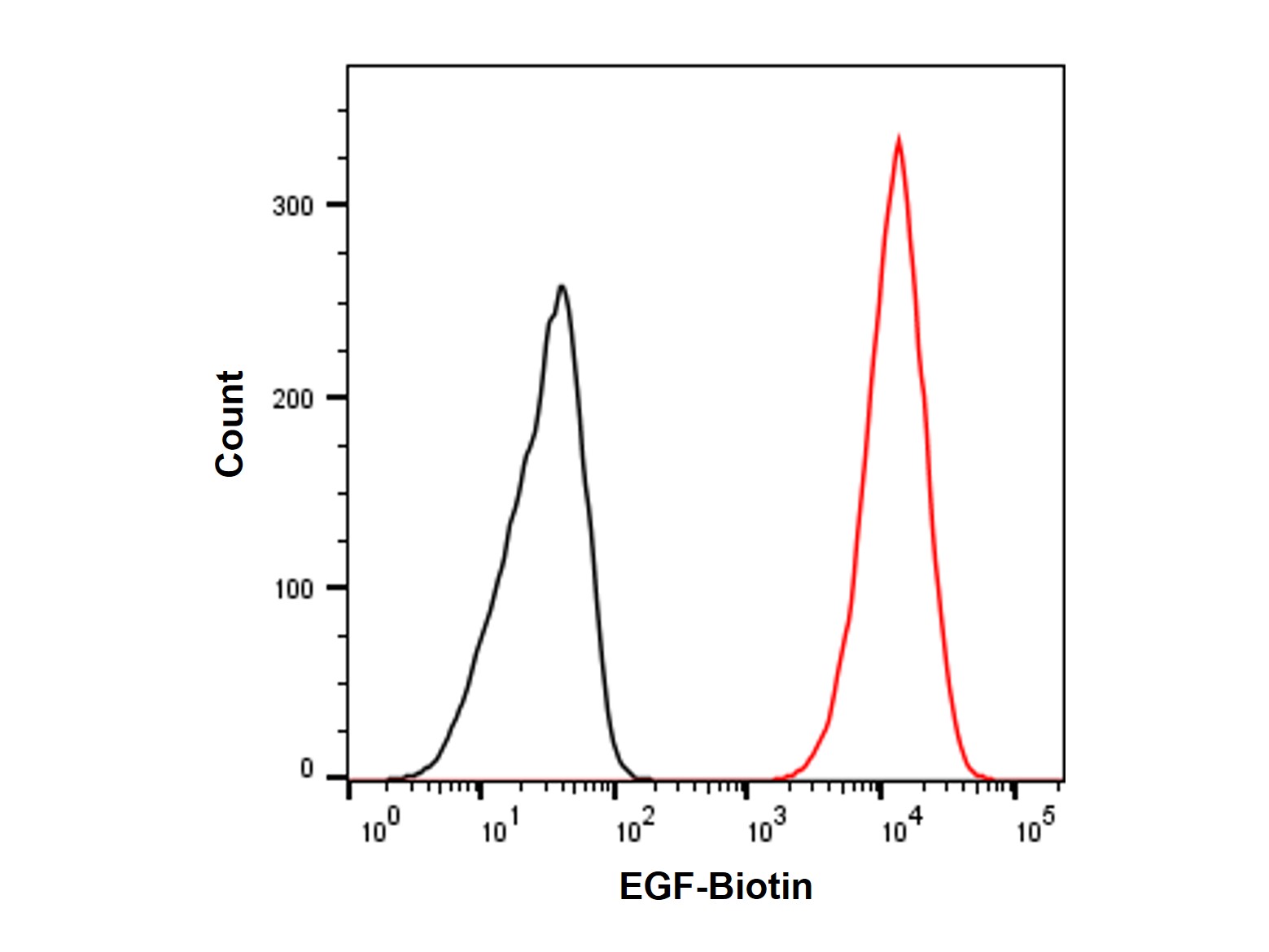
Recombinant Human EGF Protein (Biotin) (rp170191) - Flow Cytometry
Flow analysis of EGF receptors (red line) in A431 Cells. Cells were labeled with Recombinant Human EGF Protein (Biotin) (rp170191) at 1/2500 dilution for 1 hour at 4°C, and then incubated with Streptavidin protein (APC) (np169048) at a dilution of 1/400 for 30 minutes at 4°C in the dark. Unlabeled Cells (black line) were used as a negative control.
| Shape | Lyophilized |
|---|---|
| Reconstitution | This product should be reconstituted in 100μL of deionized water to make a 0.2mg/mL stock solution. Prepare working dilution on day of use. Stock solution is stable for about 8 weeks at 2-8°C as an undiluted liquid. Avoid freeze-thaw cycles. Protect from light. |
| Storage Temp | Store at 2-8°C,Protected from light |
| Shipped In | Wet ice |
| Stability And Storage | The lyophilized products should be stored at –20°C until use. Allow the product to warm to room temperature before opening. Protect from light. When stored properly, the products should be stable for at least 12 months. |
Find and download the COA for your product by matching the lot number on the packaging.
| Lot Number | Certificate Type | Date | Item |
|---|---|---|---|
| Certificate of Analysis | Jul 17, 2023 | rp170191 |

Starting at $14.90

Starting at $19.90

Starting at $19.90

Starting at $119.90

Starting at $599.90

Starting at $119.90

Starting at $69.90
| 1. Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH, Saito K, Sakamoto A, Inoue M, Shirouzu M et al.. (2002) Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains.. Cell, 110 (6): (775-87). [PMID:12297050] [10.1021/op500134e] |
| 2. Lesuisse D, Mauger J, Nemecek C, Maignan S, Boiziau J, Harlow G, Hittinger A, Ruf S, Strobel H, Nair A, Ritter K, Malleron JL, Dagallier A, El-Ahmad Y, Guilloteau JP, Guizani H, Bouchard H, Venot C.. (2011) Discovery of the first non-ATP competitive IGF-1R kinase inhibitors: advantages in comparison with competitive inhibitors.. Bioorg Med Chem Lett, 21 (8): (2224-2228). [PMID:21441024] [10.1016/j.bmcl.2011.03.003] |
| 3. Gregory, H H and Preston, B M BM.. (1977) The primary structure of human urogastrone.. International journal of peptide and protein research, [PMID:300079] |
| 4. Hommel, U U, Harvey, T S TS, Driscoll, P C PC and Campbell, I D ID.. (1992) Human epidermal growth factor. High resolution solution structure and comparison with human transforming growth factor alpha.. Journal of molecular biology, (5): [PMID:1522591] |
| 5. Furuya, M M, Akashi, S S and Hirayama, K K.. (1989) The primary structure of human EGF produced by genetic engineering, studied by high-performance tandem mass spectrometry.. Biochemical and biophysical research communications, (15): [PMID:2789514] |
| 6. Bell, G I GI and 8 more authors.. (1986) Human epidermal growth factor precursor: cDNA sequence, expression in vitro and gene organization.. Nucleic acids research, (11): [PMID:3491360] |
| 7. Lu, H S HS and 5 more authors.. (2001) Crystal structure of human epidermal growth factor and its dimerization.. The Journal of biological chemistry, (14): [PMID:11438527] |
| 8. Ferguson, Kathryn M KM and 5 more authors.. (2003) EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization.. Molecular cell, [PMID:12620237] |
| 9. Groenestege, Wouter M Tiel WM and 10 more authors.. (2007) Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia.. The Journal of clinical investigation, [PMID:17671655] |
| 10. Lu, Chafen C and 6 more authors.. (2010) Structural evidence for loose linkage between ligand binding and kinase activation in the epidermal growth factor receptor.. Molecular and cellular biology, [PMID:20837704] |
| 11. Huang, Hsiao-Wen HW, Mohan, Sepuru K SK and Yu, C C.. (2010) The NMR solution structure of human epidermal growth factor (hEGF) at physiological pH and its interactions with suramin.. Biochemical and biophysical research communications, (26): [PMID:21029725] |
| 12. Zettl, Markus M, Adrain, Colin C, Strisovsky, Kvido K, Lastun, Viorica V and Freeman, Matthew M.. (2011) Rhomboid family pseudoproteases use the ER quality control machinery to regulate intercellular signaling.. Cell, (1): [PMID:21439629] |